It’s tough enough to be a startup developing and launching innovative products. For medical device startups, there’s the added burden of operating in a highly-regulated industry.
Bill Betten, Betten Systems Solutions

[Image from Unsplash]
Unlike the consumer world – where an app or prototype can be developed in a garage (or dorm room), then publicized on YouTube and funded via Kickstarter – the regulated medical device development world requires a different level of commitment, process and demonstration of efficacy.
The topic of medical device development success could easily fill a book. This is the first in a series of articles discussing the design of innovative products in a highly-regulated environment. What are the critical elements to consider before diving into product development, and how do you pivot your process after the dive? Applying these principles to your development process will result in a faster and cheaper path to market.
Let’s focus on the definition and execution of product development activities post-funding. The critical success elements to be discussed include:
- Idea – Without it, nothing to be developed
- Process – The structure for development (The article you’re reading.)
- Plan – The blueprint
- Requirements – The details
- Regulatory/reimbursement – Critical to the medical device space
- Verification/validation – The right product doing the right thing
We’re going to take a detailed dive into each of the elements in future articles. Since you’ve already got the idea, let’s begin with process, the umbrella under which the other activities are conducted. While processes vary from organization to organization and have evolved over time, successful product developments require a certain level of structure to accomplish the goals in a timely and efficient fashion. A product development process defines the requirements and serves as a guide to ensure that products meet or exceed customer and business needs. The process also describes the stages, inputs, outputs and responsibilities associated with the subsequent activities to be performed. The process applies to all product development activities and includes significant changes in function and/or intended use on released products. Your development process also includes the Quality Management System (QMS). Many medical device companies choose to implement a quality system and have it certified to ISO 13485 to ensure consistency in this regulated industry.
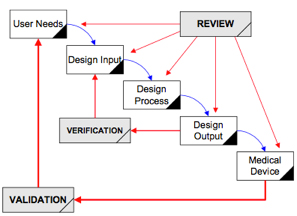
For years the FDA (and industry) reflected a “waterfall” process (image to the right) as described in the FDA Design Control guidance in 1997. This views the product development process as a set of sequential activities. Basically, requirements are developed, and a device is designed to meet those requirements. The design is then evaluated, transferred to production, and the device is manufactured. However, in the mid-2000’s, it was recognized that development is really an iterative process that incorporates interactions between a variety of stakeholders and the input of every group involved in the development process. The emphasis now is on sharing information throughout the different product lifecycle stages and those different groups, resulting in a much messier (but more realistic) interaction diagram. (See below.) It also encourages the use of Preventive Actions over Corrective Actions. In summary, the process and QMS help ensure that product development activities are conducted in a cross-functional environment. In addition to driving the initial development of the product, it also applies to the updating of a product after launch. In the regulated world where 60–80% of the development effort is associated with documentation in various forms, the process provides the framework for a successful effort. From a practical perspective, a well-managed process should enable you to answer the following questions:- What is important and why, and
- Why decisions were made
…without having to ask anyone!
Now let’s examine how the development of a project plan contributes to the successful development of your medical product>>
Bill Betten is the president of Betten Systems Solutions, a product development realization consulting organization. Betten utilizes his years of experience in the medical industry to advance device product developments into the medical and life sciences industries. Betten most recently served as director of business solutions for Devicix/Nortech Systems, a contract design and manufacturing firm. He also served as VP of business solutions at Logic PD, medical technology director at TechInsights, VP of engineering at Nonin Medical, and in a variety of technology and product development roles at various high-tech firms.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of MedicalDesignandOutsourcing.com or its employees.