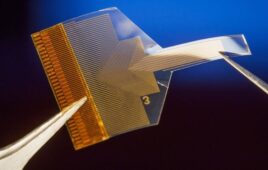
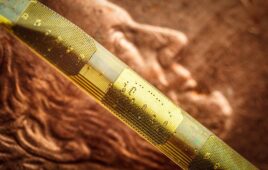
Medical device and OEM company Millar said today it inked an agreement with digital medtech company Endotronix granting it exclusive rights to its wireless MEMES sensor technology for use in neurosurgical devices.
Houston-based Millar said the sensor technology will be used to develop an at-home monitor for intracranial pressure for patients suffering from hydrocephalus, looking to improve quality of life and reduce treatment costs.
“We believe the core sensor technology and patient management platform used in the Endotronix Cordella heart failure system has applications beyond the cardiovascular space to provide clinical-grade vital sign monitoring with improved patient-physician communication. We are thrilled to partner with Millar which has an impressive history in the neurosurgical care market,” Endotronix CEO Harry Rowland said in a prepared statement.
“The implantable Endotronix sensor technology is an excellent complement to our existing catheter-based MEMS pressure sensor offerings. We have a 25-year history in sensor-enabled catheters for monitoring ICP following a traumatic brain injury. More recently we have focused on implantable MEMS platforms to expand the utility for neurosurgeons and medical researchers. We believe the Endotronix technology can provide reliable, real-time ICP measurements that will positively impact patient outcomes,” Millar CSO Simon Malpas said in a press release.