This webinar was originally broadcast on Thursday, November 10, 2016.
Click here to watch On Demand now.
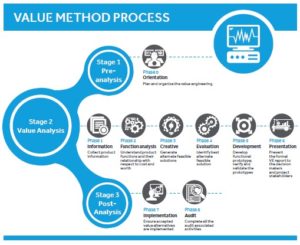
Medical device companies are continually challenged to find ways to counter market pressures and maintain product relevance. Value engineering is a systematic and function focused decision process for responding to these hurdles. Ultimately, value is improved by finding ways of cutting costs or refining the product’s functions while keeping the core product intact. Join us to learn how this standardized approach can uncover product improvements and yield results for new value.
What participants will learn:
- Why it’s important for OEMs to utilize value engineering
- Risks of not using value engineering
- Value Method process
- Design and manufacturing optimization advantages
Speakers
 Hans Mische, Sr. Director, Business Development & Strategy, Cyient
Hans Mische, Sr. Director, Business Development & Strategy, Cyient
Hans Mische has over 25 years of product development and strategy experience in the medical device and life sciences industries. He holds several patents for advancing new treatment options and has managed product development, manufacturing, collaborations, acquisitions, intellectual property portfolios and other business development activities for a number of companies. He holds a bachelor’s degree in physics from St. Cloud State University in Minnesota.
 Paul Dvorak, Moderator, Medical Design & Outsourcing
Paul Dvorak, Moderator, Medical Design & Outsourcing
Editor Paul Dvorak has several years of mechanical engineering experience and 30 years experience writing and editing technical articles and editorials. He says he’s interested in just about anything related to wind and solar. He’s an Air Force and Viet Nam veteran and even taught high-school math and science for two years before turning to engineering.
Sponsored by:



