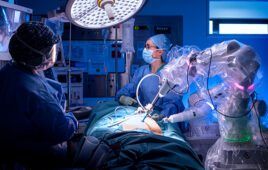
Johnson & Johnson: Ottava unveiled
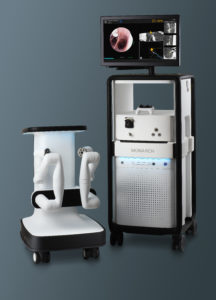
Johnson & Johnson’s existing Monarch surgery platform [Image from J&J]
J&J last year decided not to follow a 510(k) clearance pathway for what it is now calling its Ottava surgical robotic system; the company’s goal is to start first-in-human studies with the system in 2022.
“We’re very excited about Ottava. We truly think it’s going to offer next-generation digital and robotic surgery,” J&J CEO Alex Gorsky said during a Jan. 26 earnings call.
The company is planning on beginning verification and validation processes for Ottava in 2021, followed by enrollment in clinical trials for the device in 2022.
The medtech giant has been working on combining technologies that came out of its previous Verb Surgical collaboration with the Alphabet (NSDQ:GOOGL) life sciences unit Verily — as well as its $3.4 billion purchase of Auris Health and its FDA-cleared Monarch platform.
“We brought the various technologies together, we continue to do the build-outs, and we remain on track with all the timelines that we have previously committed to,” Gorsky said on Jan. 26.
The Ottava name , unveiled in November, is a tribute to the Italian word for music an octave higher. The new system is designed with six arms to provide more control and flexibility in surgery, while its arms will be integrated into the operating table. Additionally, the platform has a zero-footprint design to enable patient access, increase space in the operating room and improve workflow.
J&J’s digital ecosystem — designed to connect its surgical and robotic platforms — is set to power Ottava.
Additionally, the company’s DePuy Synthes unit announced in January that it has received FDA 510(k) clearance for the Velys system, touted as a first-of-its-kind, table-mounted ortho surgery robot with an efficient design capable of integrating into any operating room. The company said it adapts to a surgeon’s workflow, offers control they are used to and heps execute accurate bony cuts.
Velys is designed for use with the Attune total knee system and its cleared indications for use and it will become part of the broader Velys digital surgery platform of connected technologies