In the November/December 2016 issue of Surgical Products, the winners of this year’s Excellence In Surgical Products (ESP) Awards are revealed.
Day by day, we’ll also share the results here, along with an expanded version of the Surgical Products Q&A that accompanied each winner.
Category: INFECTION CONTROL
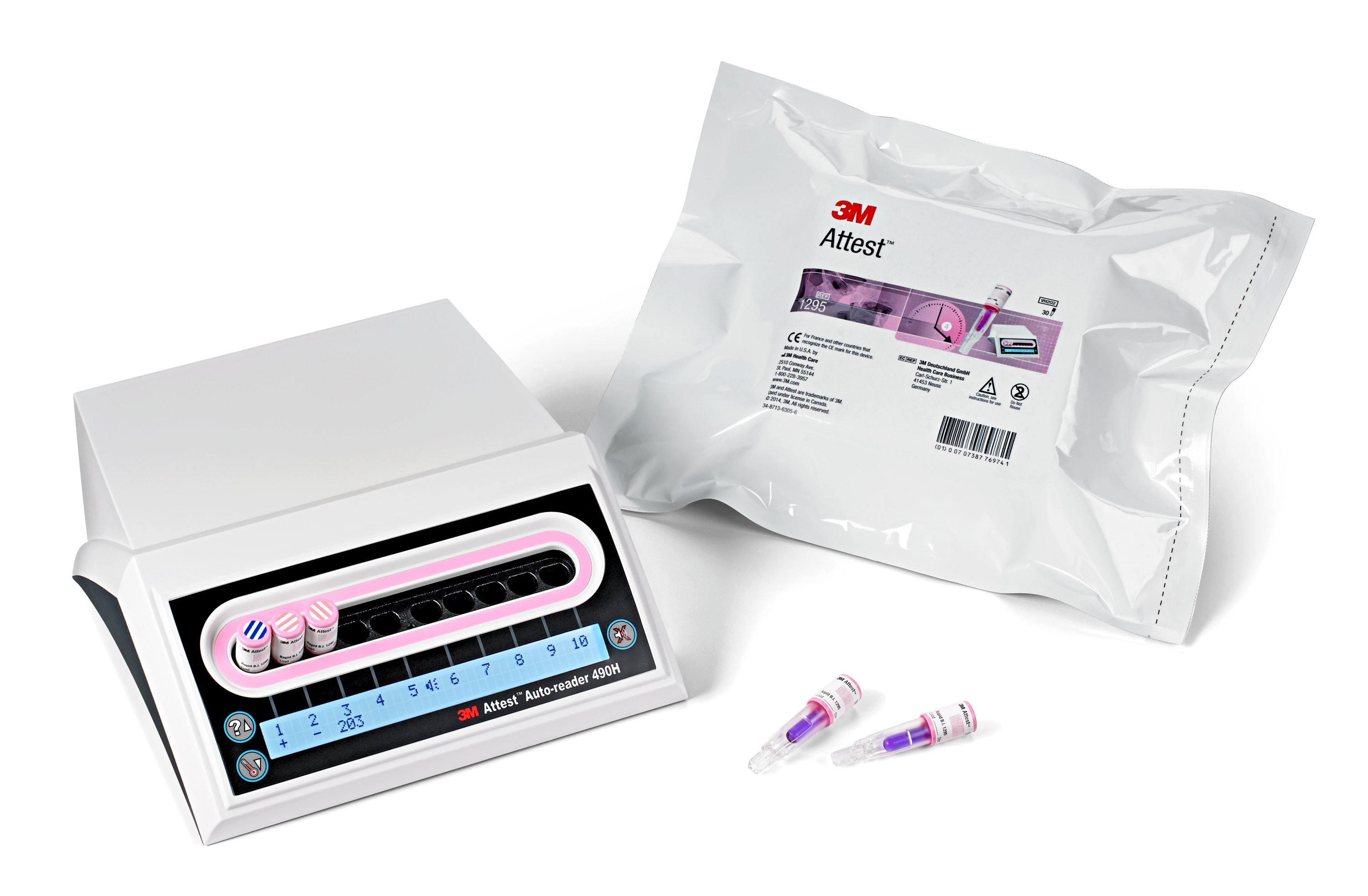
3M Attest Rapid Readout Biological Indicator System for Vaporized Hydrogen Peroxide Sterilization
Why do you think readers are interested in learning more about the product?
There’s no such thing as “good enough” when it comes to surgical procedures and the critical support systems that are in place to enable them, such as instrument reprocessing. Robust sterile processing policies and procedures go hand-in-hand with comprehensive quality monitoring systems to contribute to the best possible patient outcomes.
For vaporized hydrogen peroxide sterilization loads, the confidence of a biological indicator result is now available in just four hours, versus the standard 24 hours. Four-hour results with the 3M Attest give sterile processing personnel the information they need when they need it, while there is still time to act. In the event of a sterilization process failure, this speed makes it possible to alert surgical personnel to a sterilization process failure while a patient is still in the OR, or perhaps even before the instrument set leaves sterile storage.
With faster results, it’s practical to standardize on rapid readout biological indicators across sterilization modalities, whether it is steam, ethylene oxide, or vaporized hydrogen peroxide. Faster biological results can also help make it easier and more practical to practice every load monitoring—a biological indicator in every sterilization load—which provides patients with the highest and most consistent quality of care.
(Image credit: 3M)
How does this product benefit the surgical team and aid in better outcomes?
Proper cleaning and decontamination, followed by sterilization with a biological indicator in every load, provides the utmost level of assurance that each sterilization cycle was efficacious. Monitoring every load provides consistency for patients with a true test of lethality in every sterilization load, consistency for sterile processing staff with less room for error because the same process is used for all loads, and robust quality assurance and risk management, because when each load is monitored with a rapid biological indicator, you minimize risks associated with an undetected sterilization failure. And that is good for everyone.
As I’ve heard many Sterile Processing leaders say, “If it were my family member being prepped for surgery, I would want their instruments to be monitored by a biological indicator. And everyone is someone’s family member.”
What technical innovations does the product encapsulate and apply to the OR?
The 3M Attest Rapid Readout Biological Indicator System for Vaporized Hydrogen Peroxide Sterilization was born in the same 3M lab where the first self-contained biological indicators (1971) and the first rapid readout biological indicator (1991) were invented. The scientific legacy of many chemists, biologists, microbiologists, and engineers from several disciplines from across the 3M technical community made it possible for over 850 million cycles to be monitored by Attest biological indicators all around the world.
All Attest rapid readout biological indicators are designed to indicate whether bacterial spores remain alive after exposure to the specified sterilization process. They do this by detecting the activity of germinating spores, which in turn activates an ingredient in the growth medium that results in fluorescence. The fluorescence is read by the 3M Attest Auto-reader, which provides a clear positive or negative result, eliminating the need for the technician to interpret a subjective color change or attempt to decipher a change in turbidity. A negative (-) result for a processed biological indicator indicates a successful sterilization process. A positive (+) result for a processed biological indicator indicates a sterilization process failure.
By partnering with leading instrument tracking companies, we can help bring real-time results right to your recordkeeping system for more streamlined record keeping and improved accuracy. The latest generation of 3M Attest Auto-readers is designed to directly connect to leading instrument tracking software systems through a facility’s Ethernet. As more and more healthcare facilities and entire healthcare systems adopt this type of recordkeeping software, it’s important to consider data integration and reliability when making a purchase decision.




