The 2018 Excellence in Surgical Products Awards will be presented in the November/December issue of Surgical Products. Leading up to the publication of that issue, nominees will be featured on the Surgical Products website.
Category: Orthopedics
Product description and innovation synopsis:
Designed to deliver comfort and precision, the new Hall MicroFree Cordless Small Bone Power System features cordless, battery-powered, pencil-grip instruments that can be used for most small bone procedures of the foot, ankle, hand, and wrist. It’s time to cut the cord on your small bone power.
• Cordless handpiece is designed to improve hand positioning and maneuverability
• Well-balanced ergonomic handpieces are designed to perform comfortably in surgeons’ hands
• Adjustable pencil grip designed for precise cutting
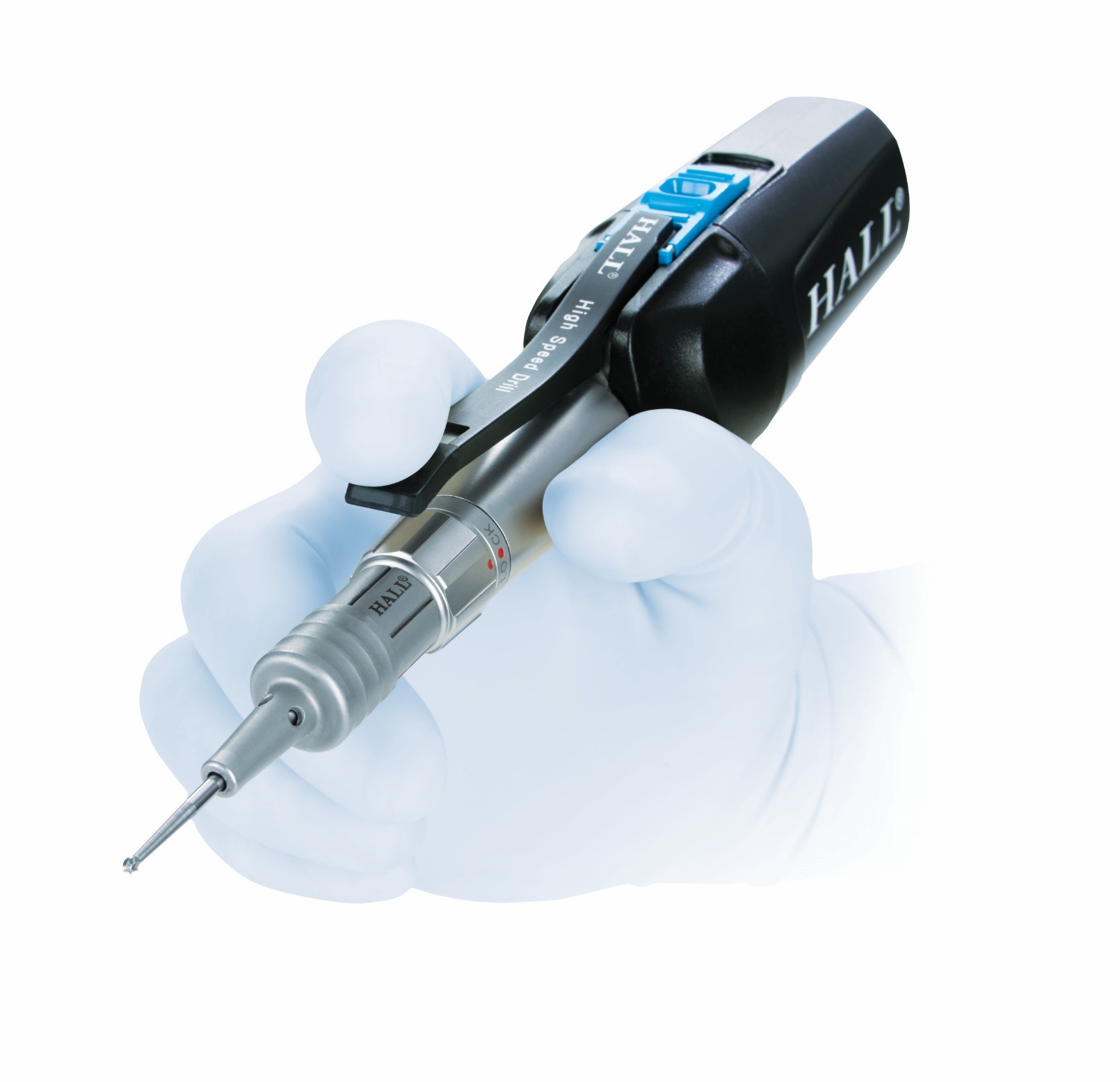
(Image credit: CONMED)
What sets this product apart from others available in the industry?
When CONMED asked surgeons how we could make their jobs easier, the answer was clear: cordless power handpieces that provide freedom of motion without sacrificing power. It was clear these handpieces must be comfortable, ergonomic, well-balanced, and deliver the same power surgeons were accustomed to with corded systems. MicroFree went through extensive design testing with leading orthopedic surgeons. Using their feedback, the center of gravity was adjusted to improve balance and control while reducing muscle fatigue.
This system was featured in Outpatient Surgery Magazine’s “A Dozen Hot Orthopedic Products from AAOS 2018.”
How does this product benefit the surgical team and aid in better outcomes?
By eliminating unsecured power cords from compromising the sterile field, the MicroFree System may reduce those potential risks that can contribute to surgical site infections (SSIs). Both the UL-approved batteries and handpieces of the MicroFree System are autoclavable, eliminating the need for more expensive STERRAD battery sterilization, allowing the batteries, handpieces and attachments to be sterilized together, simplifying the process, saving OR staff time and energy. The MicroFree handpieces are rated IPX 6 and IPX 8 to help protect the system against water damage and the power units are UL-Approved.




