Mentor Graphics announced the newest version of its FloTHERM product, the computational fluid dynamics (CFD) software solution. The FloTHERM product was developed to quickly identify potential thermal issues early in the design process to avoid expensive late stage re-design and warranty costs arising from thermal failures in the field. The newest version of the solution delivers an automated method to calibrate simulation models to match transient thermal measurements recorded with the Mentor Graphics T3Ster hardware. As a result, the FloTHERM product can help maximize simulation data accuracy and provide additional insight into product reliability—a necessity for the automotive, aerospace and electronics products industries.
The new FloTHERM product can convert a simulated transient thermal response into a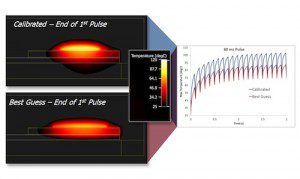 structure function curve using the same mathematical process utilized by the T3Ster product.The new version uses optimization methods to change the model inputs and drive the simulation structure function toward the experimental structure function until a match is achieved. This match indicates that the FloTHERM model is fully calibrated and will respond correctly and accurately in any transient application. Package manufacturers can now certify supply chain models by providing evidence about the thermal performance of the component in customer applications, along with the model itself.
structure function curve using the same mathematical process utilized by the T3Ster product.The new version uses optimization methods to change the model inputs and drive the simulation structure function toward the experimental structure function until a match is achieved. This match indicates that the FloTHERM model is fully calibrated and will respond correctly and accurately in any transient application. Package manufacturers can now certify supply chain models by providing evidence about the thermal performance of the component in customer applications, along with the model itself.
Additional features in the new FloTHERM product include joule heating, FloMCAD Bridge enhancement,localized grid spaces, and a DCIM Software Development Kit.
Mentor Graphics
mentor.com




