TDK Corporation announces the introduction of TDK-Lambda’s CUS350M medical and ITE certified 350W power supplies. Requiring no external airflow, the series is suitable for hospital, clinical, dental and home based medical applications as well as professional broadcast, test, measurement and audio equipment where low audible noise is demanded.
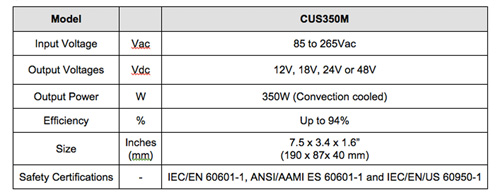
Carrying a 3-year warranty, the CUS350M series is currently available with 12V, 18V, 24V, or 48V outputs with a user adjustment of +/-5% to accommodate non-standard voltages. The series can reliably provide up to 350W output power (convection cooled) at 40oC ambient, and 175W at 70oC. With efficiencies of up to 94%, an average rating of 87% and a standby power consumption of less than 0.5W, the units comply with the European ErP Directive.
The units are enclosed in a compact 190mm x 87mm x 40mm (7.5” x 3.4” x 1.6”) package and will operate from a wide 85 to 264Vac input. The fully featured “/F” suffix models include a 5V 0.5A standby output, a 12V 0.3A auxiliary output, remote sense, an isolated DC Good signal and remote on/off function. Input to output isolation is 4,000Vac (2 x MOPPs) and 1,500Vac (1xMOPPs) input to ground and output to ground.
The CUS350M power supplies are certified to IEC/EN 60601-1, ANSI/AAMI ES 60601-1 and IEC/EN/US 60950-1 and are CE marked for the Low Voltage and RoHS2 Directives. The units also comply with EN 55011-B, EN 55022-B and FCC Class B for conducted emissions (Class A radiated), and meet the IEC 61000-4 immunity and IEC 61000-3-2 harmonic current standards.
More information can be obtained at the following TDK-Lambda Americas website, http://www.us.tdk-lambda.com/lp/products/cus-m-series.htm, or by calling 800-LAMBDA-4. Product availability for the CUS350M series can be found via the link to TDK-Lambda’s distributor network (see “Check Distributor Stock”) at http://www.us.tdk-lambda.com/lp/.
Major applications
Medical, test and measurement, broadcast and audio equipment
Main features and benefits
- 350W output power without external airflow
- Medical and ITE certifications
- Efficiencies of up to 94%
- Fully featured (/F suffix)
TDK-Lambda Corporation
www.tdk-lambda.com