7D Surgical announced that it has received both 510(k) clearance from the U.S. Food and Drug Administration (FDA) and a medical device license from Health Canada enabling the North American commercial launch of its innovative Machine-vision Image Guided Surgery (MIGS) system for spine surgery, the 7D Surgical System.
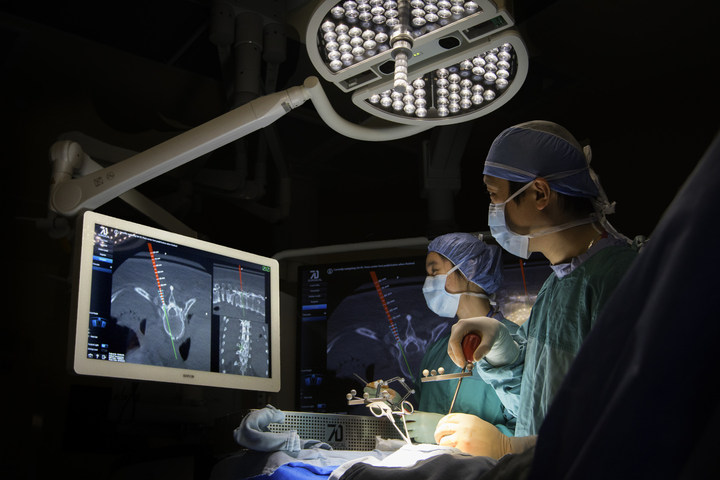
The 7D Surgical System employs cutting-edge 3D optical technologies and machine vision algorithms to eliminate the long-standing barriers to adoption of existing surgical navigational platforms. This new technology can easily register spinal surgery patients automatically using only visible light. Unlike time-consuming conventional image guided surgery (IGS) systems that depend on intraoperative radiation, this new platform can achieve an incredibly fast surgical workflow for spine procedures.
“When navigating the spine, surgeons traditionally have had two time-consuming and expensive IGS options: systems that rely on intraoperative radiation emitting devices or systems that utilize laborious manual point matching techniques,” says Beau Standish, Chief Executive Officer of 7D Surgical. “We believe the inefficiencies of these systems have limited the adoption of IGS in spine procedures to less than 20 percent. 7D Surgical’s MIGS system has now removed these barriers, providing surgeons and their hospitals with a superior product option.”
(Image credit: 7D Surgical)
The 7D Surgical System enables near-instantaneous Flash Registration of the patient’s anatomy. “Guided by our product philosophy of ‘surgeons designing for surgeons,’ we have achieved an unprecedented entire workflow time of less than 20 seconds for de novo spinal registration, unheard of in the spinal IGS world where such registration can interrupt surgery for up to 30 minutes,” says Dr. Victor Yang, president and chief scientific officer of 7D Surgical, senior scientist at Sunnybrook Research Institute, and staff neurosurgeon at Sunnybrook Health Sciences Centre in Toronto, where prototype MIGS technology has been used in more than 160 patients in clinical trials.
The MIGS navigation technology is embedded in an onboard overhead surgical light, which eliminates line of sight frustrations in the operating room, and is manifested in simplified yet powerful software, which is controlled by the surgeon using only a foot pedal. “Our surgeons don’t rely on non-sterile personnel to operate the technology. Our surgeons are in total control,” says Yang.
“Image guided surgery technology has finally caught up to the needs of a practicing spine surgeon,” says Dr. Frank Cammisa, chief mmeritus of the Spine Service at Hospital for Special Surgery in New York City. “7D Surgical’s new MIGS system appears to provide a faster, radiation-free alternative to existing options. It could be an important new tool in expanding the use of IGS in spine procedures. The 7D Surgical System reduces the overall cost and footprint required to navigate the spine. It’s a win-win for the surgeon and the hospital.”
With both U.S. and Canadian regulatory authorization, 7D Surgical has commenced execution of its North American commercialization strategy. “We are delighted to have achieved these regulatory milestones in line with our expectations and planning,” says Standish. “We are confident that in demonstrating the speed and efficiency of our MIGS system, we will convince more surgeons to employ IGS in their spine procedures.”




