(Credit: PR Web)
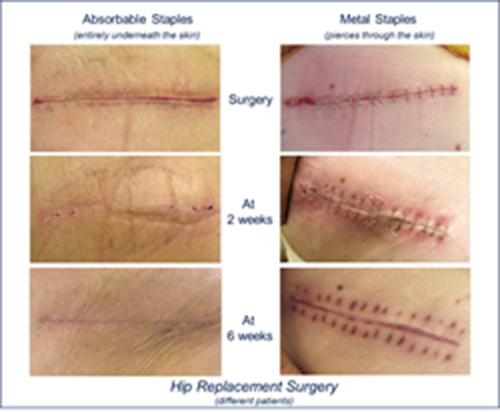
Incisive Surgical, Inc., the developer of a revolutionary INSORB® Absorbable Subcuticular Skin Stapling Closure Modality, announced today the results of a new online nationwide survey conducted by SurveyMonkey. After viewing post-operative surgical photos of two hip replacement procedures closed with traditional metal skin staples and INSORB® Absorbable Subcuticular Staples, 94% of the 615 respondents preferred the Absorbable Staple closure. Nearly 82% of the respondents would be “very” or “somewhat” upset and disappointed if their physician did not discuss their skin closure method options with them prior to their surgical procedures.
The universal Patient’s Bill of Rights states that patients have the right to know their treatment options and to take part in decisions about their care. More and more patients are exercising these rights to select more patient-centric surgical solutions, and to demand that they be made fully aware of their options and participate in any decision-making.
The unique INSORB Absorbable Skin Stapling Technology is a patented new skin closure technology that places an absorbable staple entirely underneath the top layer of skin to close surgical incisions. Patients and clinicians now have a more comfortable, convenient and cosmetic choice for rapid skin closure. This proprietary device been recognized with several national awards, including the 2006 Wall Street Journal Technology Innovation of the Year Award for medical devices and the 2014 Global Frost & Sullivan Award for Customer Value Leadership. Incisive Surgical is the only company in the world that has commercialized this technology. Over 1,900,000 INSORB Absorbable Skin Staplers have been sold throughout the world since the product launch in January 2005, and the Company estimates that over 100,000 cesarean sections performed in the United States were closed with the INSORB Stapler in 2015.
The use of metal skin staples results in rapid incisional closures, but each metal staple pierces and punctures the skin in two places. This unnecessary additional trauma creates more pain, may compromise healing and the cosmetic result, and may cause increased complications. In addition, metal skin staples require removal which is inconvenient, painful and costly. The INSORB Absorbable Skin Stapler offers the speed of metal skin staplers with the cosmetic results and comfort of absorbable sutures. The INSORB Stapler uses bioabsorbable staples that are placed underneath the top layer of skin and dissolve in the body within a matter of months. The staples, which are comprised of the same material as the leading bioabsorbable sutures, break down naturally, thereby eliminating the anxiety, pain, cost and inconvenience of post-operative metal staple removal.
“These results of this SurveyMonkey survey are profound,” said John L. Shannon, Jr., president and chief executive officer of Incisive Surgical. “These findings confirm that patients have high expectations regarding their surgical care, and expect that clinicians will consult with them regarding their options prior to any surgical procedure and provide patient-centric alternatives. It is our opinion that, in the 21st Century, metal skin staples are barbaric and should be obsolete for routine skin closure. The results of this survey clearly confirm this belief.”




