A new nanofiber-on-microfiber matrix could help produce more and better quality stem cells for disease treatment and regenerative therapies.
A matrix made of gelatin nanofibers on a synthetic polymer microfiber mesh may provide a better way to culture large quantities of healthy human stem cells.
Developed by a team of researchers led by Ken-ichiro Kamei of Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS), the ‘fiber-on-fiber’ (FF) matrix improves on currently available stem cell culturing techniques.
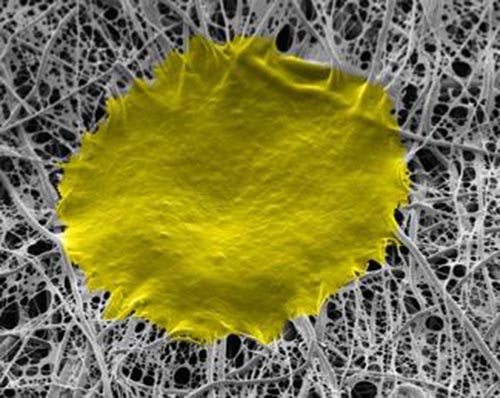
These are human stem cells that grew on the ‘fiber-on-fiber’ culturing system. (Credit: Kyoto University iCeMS)
Researchers have been developing 3D culturing systems to allow human pluripotent stem cells (hPSCs) to grow and interact with their surroundings in all three dimensions, as they would inside the human body, rather than in two dimensions, like they do in a petri dish.
Pluripotent stem cells have the ability to differentiate into any type of adult cell and have huge potential for tissue regeneration therapies, treating diseases, and for research purposes.
Most currently reported 3D culturing systems have limitations, and result in low quantities and quality of cultured cells.
Kamei and his colleagues fabricated gelatin nanofibers onto a microfiber sheet made of synthetic, biodegradable polyglycolic acid. Human embryonic stem cells were then seeded onto the matrix in a cell culture medium.
The FF matrix allowed easy exchange of growth factors and supplements from the culture medium to the cells. Also, the stem cells adhered well to the matrix, resulting in robust cell growth: after four days of culture, more than 95 percent of the cells grew and formed colonies.
The team also scaled up the process by designing a gas-permeable cell culture bag in which multiple cell-loaded, folded FF matrices were placed. The system was designed so that minimal changes were needed to the internal environment, reducing the amount of stress placed on the cells. This newly developed system yielded a larger number of cells compared to conventional 2D and 3D culture methods.
“Our method offers an efficient way to expand hPSCs of high quality within a shorter term,” writes the researchers in their study published in the journal Biomaterials. Also, because the use of the FF matrix is not limited to a specific type of culture container, it allows for scaling up production without loss of cell functions.
“Additionally, as nanofiber matrices are advantageous for culturing other adherent cells, including hPSC-derived differentiated cells, FF matrix might be applicable to the large-scale production of differentiated functional cells for various applications,” the researchers conclude.




