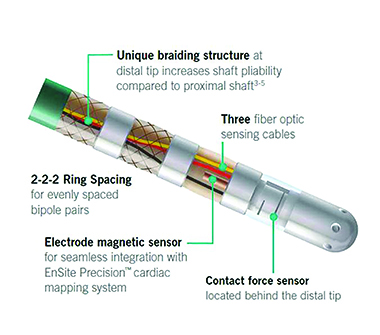
The new TactiCath contact ablation catheter unites EndoSense and St. Jude Medical technologies that Abbott acquired, marrying fiber optics, flexibility and 3D mapping.
If you want your grilled cheese to cook faster, you press it into the pan with your spatula. That’s contact force, the principle behind Abbott’s TactiCath contact force ablation catheter, according to Srijoy Mahapatra, Abbott’s VP of medical, clinical and scientific affairs.Abbott acquired TactiCath with the purchase of St. Jude Medical this year. (St. Jude acquired Swiss company Endosense in 2013.) The latest version of the TactiCath, which won CE Mark approval in the European Union in May, unites the Endosense device with the EnSite Precision cardiac mapping system developed by St. Jude.
Although cardiac tissue ablation is far more sophisticated than whipping up a grilled cheese, the physics of the issue are the same. The more force the surgeon uses when the catheter is touching the tissue, the bigger the lesion. “Too much force can be dangerous; too little force means no lesion,” Mahapatra explained.
The new TactiCath is sensor-enabled and emphasizes accuracy and precision through dual impedance and magnetic technologies designed to precisely model the heart to determine where to apply optimal contact force.
TactiCath is built on Abbott’s FlexAbility catheter platform, featuring an ergonomic handle-shaft combination designed for reach, maneuverability and 1:1 torque.
“A big change from the old fiberoptic system to the new one is we moved it over to a catheter that’s much more maneuverable,” Mahapatra said, noting that Abbott is planning the same for all future catheters.
Fiberoptics bring contact force information into the hands of the surgeon, using a Fabry–Pérot interferometer of three fiberoptic lines and three glass wires. Light emitted from the system travels through the TactiCath toward the tip and is reflected back and forth many times as it enters the force sensor’s micrometric cavities. The differing path lengths of the resulting beams create interference patterns; when force is applied, the cavity length changes and thus the interference pattern. Interference based on the signals returned by the three optic fibers are analyzed to determine the three cavities and the exact computation of both magnitude and orientation of the contact force. The fiberoptic technology works both axially and laterally, and the size of the fiberoptic lines allows them to be only 4 mm from the distal tip of the catheter (compared with competitor products with lines as far back as 9 mm, according to Abbott).Mahapatra noted that although the force-sensing system provides high accuracy, it wasn’t as good on its own as it could be. Physicians wanted another form of data to create a more detailed heart model – hence the introduction of the Ensight Precision mapping element. Magnetic sensors act as a coordinating system built under the operating table, coordinating with magnets within the catheter to provide tracking.
“It adds another layer of feedback to the system,” Mahapatra said.
Non-fluoroscopic cardiac mapping systems are designed to provide the required spatial anatomical information in combination with local electrical information. EnSite is an open system with 3D visualization, enabling both magnetic and impedance (resistance) data. Fusion algorithms and respiratory compensation allow for model-guided therapy with real-time non-fluoroscopic visualization of intracardiac catheters within registered 3D CT/MRI images.
The full system enables automated guidance of lesion marking, verification of the ablation catheter stability, the ability to automatically record the precise location of the catheter tip during radiofrequency energy application, the capability to add mapping points based on contact force and the ability to review and identify any potential gaps by viewing specific lesions from the display list.
“The goal in developing the TactiCath was to provide the most innovative solution for treating atrial fibrillation and lead the way in clinical outcomes for patients with cardiac arrhythmias – even during long and complex ablation procedures,” Mahapatra said. “Integration with the EnSite Precision cardiac mapping system provides an unprecedented opportunity to help patients suffering with atrial fibrillation.”
Abbott is launching the new TactiCath in select markets in Europe, with a full market release expected in the third quarter this year. The company is also pursuing approval in the U.S.