Acclarent, Inc., part of the Johnson & Johnson Medical Devices Companies and a leader in developing minimally-invasive Ear, Nose & Throat (ENT) technologies, announced the U.S. commercial launch of TruDi, a real-time, three-dimensional (3D) navigation system for ENT procedures performed in the operating room and surgeon office. The TruDi Navigation System recently received 510(k) clearance from the FDA. First cases were completed Monday, April 16 at two leading U.S. healthcare facilities.
TruDi is an electromagnetic image-guided navigation system designed to offer ENT surgeons an accurate, simple and reliable option for their endoscopic sinus surgery procedures. It is intended for use during intranasal and paranasal image-guided navigation procedures for eligible patients requiring sinus surgery. TruDi enables the use of RELIEVA SPINPLUS NAV, the first 3D navigation-enabled Balloon Sinuplasty System for the treatment of chronic sinusitis.
“TruDi enables precise localization of the surgical device relative to the preoperative CT scan, facilitating the surgeon’s understanding of the complex anatomy of the narrow spaces of the paranasal sinuses,” says Dr. Martin J. Citardi, Professor and Chair of the Department Otorhinolaryngology-Head and Neck Surgery at McGovern Medical School at The University of Texas Health Science Center at Houston. “The system incorporates microsensor technology, innovative tracking hardware and software tools enabling the surgeon to easily and accurately confirm instrument placement during minimally invasive sinus procedures.”
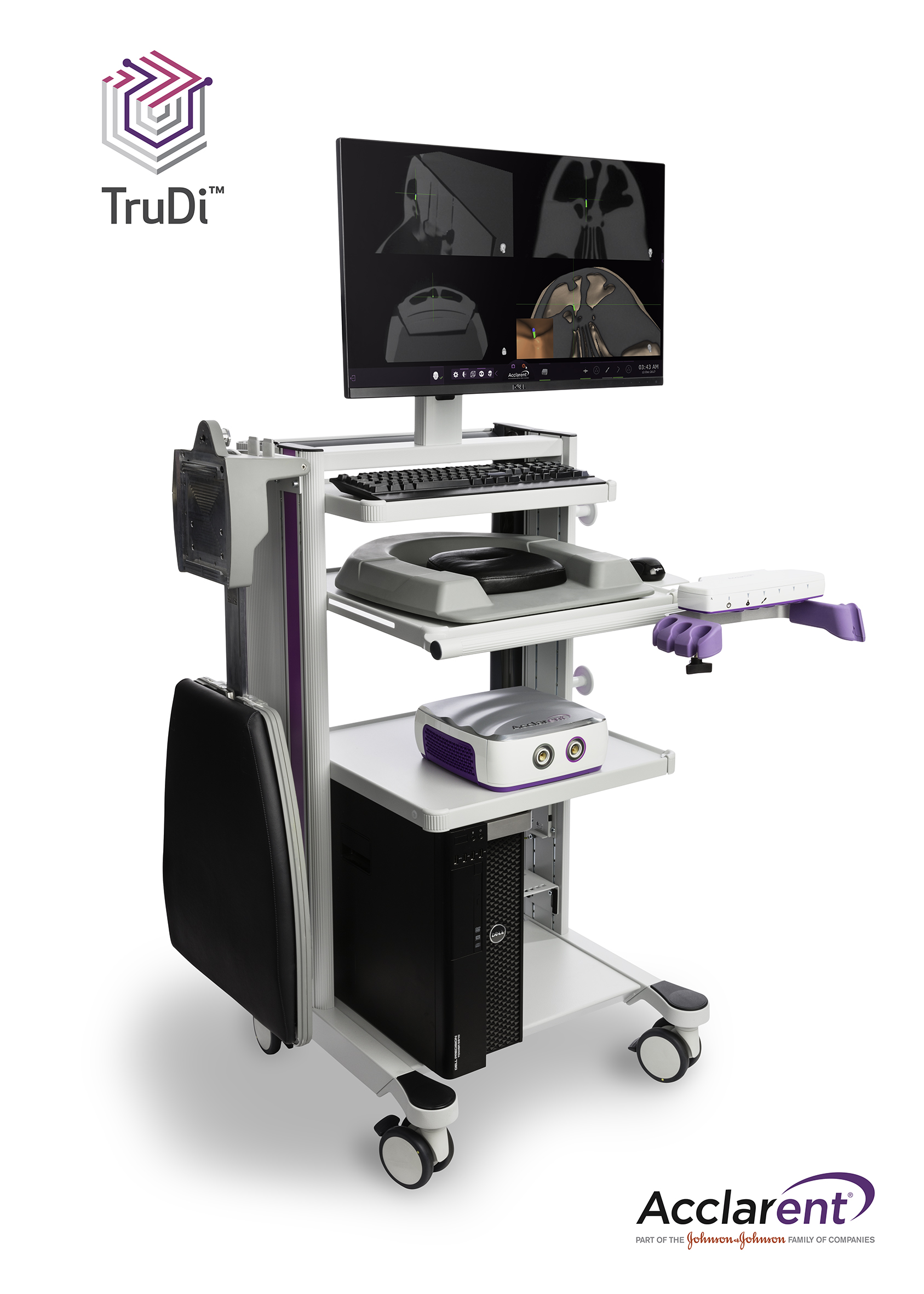
Acclarent developed TruDi in collaboration with Biosense Webster, Inc., the global leader in the science of diagnosing and treating heart rhythm disorders. TruDi leverages Biosense Webster’s advanced imaging system which utilizes electromagnetic technologies to help electrophysiologists navigate the beating heart by generating an accurate map, and pinpoints the exact location and orientation of catheters used during diagnostic and therapeutic procedures.
“With the launch of TruDi, Acclarent builds on its legacy of pioneering minimally invasive treatment options for patients suffering from Chronic Sinusitis and Eustachian Tube Dysfunction,” says Shlomi Nachman, Company Group Chair of Johnson & Johnson Medical Devices Cardiovascular & Specialty Solutions. “We’re extremely proud of this advancement and the potential it has to transform ENT procedures.”
After pioneering Balloon Sinuplasty as a minimally invasive treatment option for chronic sinus sufferers, Acclarent went on to develop the first Eustachian Tube Dysfunction intervention with ACCLARENT AERA. With TruDi, Acclarent has now taken another step in advancing ENT paranasal sinus surgery with innovative 3D navigation technology.




