Additive manufacturing (AM), or 3D printing, receives a lot of attention as the next big disruptor for the medical field and medical related applications. Deservedly so, as the medical industry accounts for around 17 percent of the total AM market1. Despite the promise of AM, many in the medical industry don’t see it as a production level technology. Instead, AM is viewed as something that lives in the lab serving or applied to a few small-scale applications, such as producing hearing aids or dental crowns.
In reality, there are a growing number of AM applications from prosthetics to prescription capsules. Perhaps one of the most promising and immediate areas is in orthopedic restorations such as creating personalized cutting guides, knee or spinal implants. At the 2018 Implants Conference in Paris, Ali Madeni, CEO and founder of Avicenne Medical, spoke about the growth potential of AM for medical purposes, citing that Avicenne analysts expect orthopedic applications to achieve around 20 percent growth per year from 2016 -20212.
Improving Surgical Precision
AM is widely used for medical prototyping and creating anatomical models. However, surgical instrumentation and cutting guides have become significant application areas3. Smith and Nephew creates 3D printed cutting guides that simplify the surgical steps for knee arthroplasty, which are used in one in four Smith & Nephew knee replacement surgeries4.
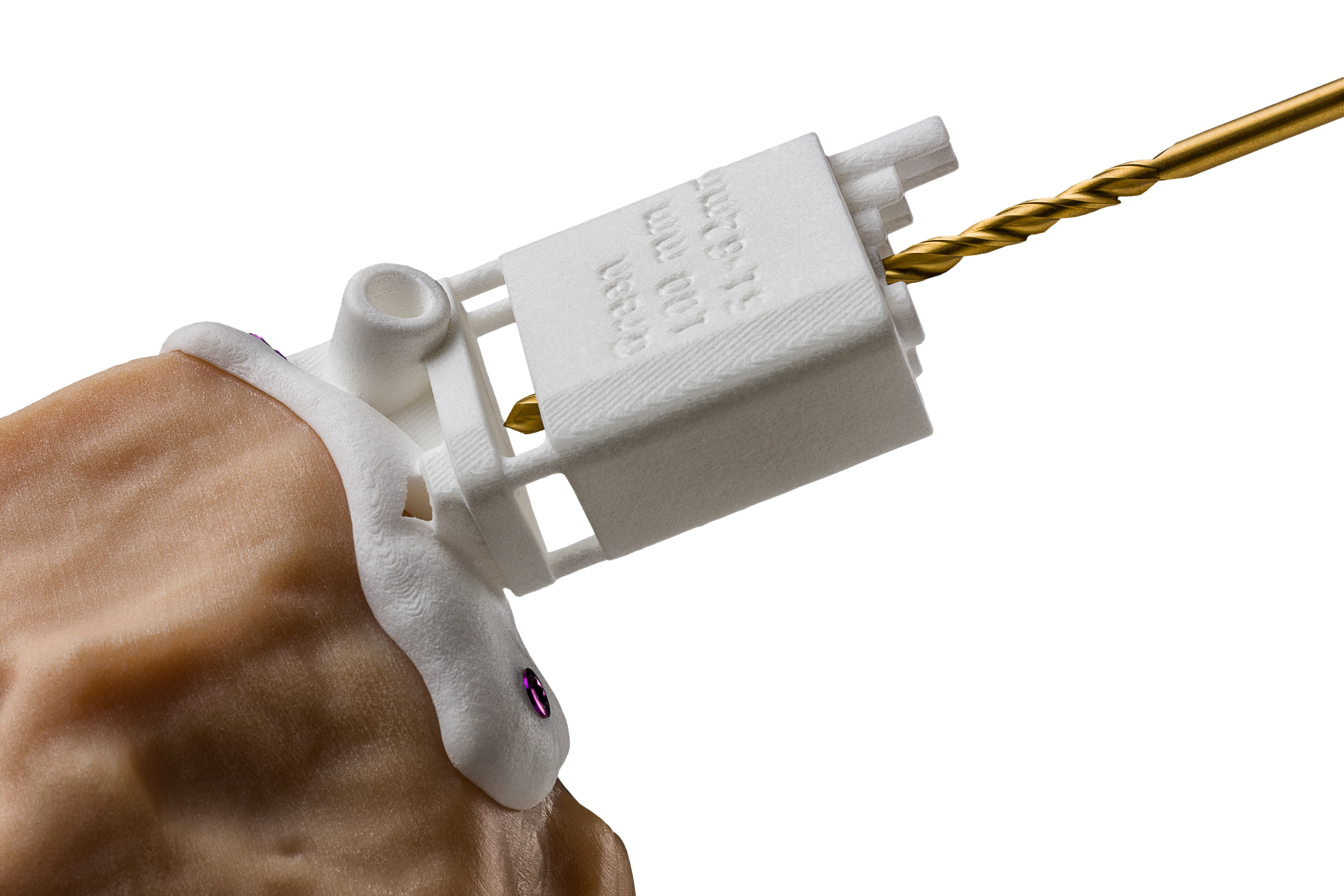
Figure 1. BodyCad’s 3D printed cutting guides simplify the surgical steps for knee arthroplasty, used in knee replacement surgeries.
Unlike mechanical parts or equipment, there is no standard for the parts that make up the human body. For example, almost five million Americans5 have undergone knee arthroplasty. Their quality of life and ability to make basic movements on their own after receiving a knee replacement depends greatly on the level of precision of their surgery. While generic hand tools are the standard for performing surgical implantation, AM has emerged as a cost effective and precise means for a mass customized approach.
Another example, Bodycad, which makes custom orthopedic restorations, has employed 3D imaging to accurately map out personalized images of a patient’s knee. Bodycad then uses 3D printing to create precise custom cutting tools (Figure 1) and prosthetic replacements (Figure 2). Lab data shows that these cutting guides are more precise than handheld tools, and just as precise as robotic assisted systems but without the capital equipment cost of a robot6.
These examples of AM produced knee cutting guides can save over $900 per operation7, a direct result of reduced surgical time by up to three weeks and 21 steps eliminated from surgery8. While cutting back on surgical steps can help reduce operating room time, thus cost, the process can also lower cost per surgery by lessening the amount of instrumentation needed as compared to standard total knee arthroplasty. More importantly, it allows surgeons to preserve bone and tissue by being more precise, improving the long-term results for the patient.
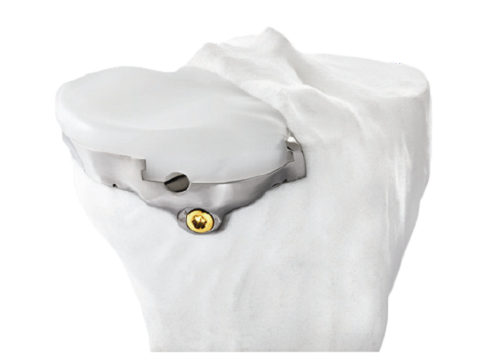
Figure 2. Bodycad uses 3D printing to create precise prosthetic replacements.
Additive’s Clinical Advancements
The growing use of AM for prosthetics opens new opportunity to create a personalized surgical experience that addresses a patient’s unique anatomical features. For example, it allows engineers to simplify processes, such as adding a porous surface layer to implants to ensure better osteointegration and improve how a patient’s bone grows into it. Traditional methods included a secondary step of spraying on this porous surface, while additive methods allow this complex design to be made with the rest of the part. This eliminates risk of delamination between the coating and the solid portion of the implant.
While it’s still an area the medical field is exploring, AM allows for the development of patient-tailored parts through 3D data obtained from MRI, CT or CAD9. Since AM produced parts, like spinal implants (Figure 3), can be made as a single unit part (instead of having multiple components), the structural integrity of the part is much greater than those produced using traditional methods. A complex implant produced using AM isn’t isolated to the R&D lab either. There are several cases of additive implants with longer-term clinical history. For example, more than 100,000 3D printed hip cups have been implanted in patients, with 10 years of clinical history10.

Figure 3. 3D printed spinal implants can be made as a single unit part instead of having multiple components, increasing structural integrity over parts produced using traditional methods.
How 3D Printing Stacks Up to Traditional Machining
In medical parts production, choosing a manufacturing method always depends on the application and ability to redesign a product. Generally, you’re not able to design a device or instrument for a traditional subtractive method and then decide to additively produce it one-to-one, especially not from a cost-effective standpoint. In most cases, a redesign for true functionality is beneficial to utilize the potential of AM. At the most basic level, AM is cost effective in design when it’s used to accomplish three things:
- Create a complex design not feasible using traditional methods
- Address a unique or custom patient need
- Reduce the number of parts and make an integrated design
Spinal implants are one example that demonstrates the ability to use AM methods to innovate faster with less capital investment by optimizing production to one whole part. Within a spinal product family, there are a variety of surgical approaches which require multiple implant designs, all needing their own traditional machining set up for production. With AM, one machine can produce many different designs, eliminating the need for multi-tooling and decreasing the large capital investment needed to set up production. This type of application enables smaller companies to gain entrance and compete in the market.
AM has great potential to significantly reduce costs, especially when it comes to part consolidation. In the case of medical instrumentation, AM has proved to drastically reduce the number of parts, with the potential to shorten production time—even eliminating distribution barriers by having parts made onsite. When it comes to comparing additive versus traditional subtractive methods of manufacturing, it always depends on what you’re making, what you’re trying to achieve and the flexibility of the supply chain model.
An Additive Future
Medical device makers were some of the earliest adopters of AM back when the technology was first available in the 1980s for prototyping purposes. Since then, applications have gone far beyond prototyping, with some device makers making several thousand of printed devices a month, bringing AM into “production level” territory. If orthopedic device manufacturers are not looking at AM, they are behind.
The industry has already seen regulatory activity pick up in regard to AM, with the more than 100 FDA cleared additively manufactured devices currently on the market. AM processes are subject to the same level accuracy and material contamination risks of any manufacturing approach, which means companies have to invest time and money into R&D before AM parts are ready for market, just as they would with conventional manufacturing techniques. The good news is that R&D efforts occurring over the past few decades are supporting the technology, allowing it to gain traction in an increasing number of applications. In response, the FDA has put significant focus on providing a regulatory pathway in an attempt to keep up with the rapid pace of adoption.
Those that are first to market with a new technology are often the most successful, whatever the industry or field. The medical industry is not only well ahead of the game when it comes to AM adoption and application, it is positioned to rapidly increase its investment in the near future.
References:
1Deloitte: 3D opportunity in medical technology
2Avicenne Medical: The worldwide orthopedic Contract Manufacturing market report 2016-2021 and Top 100 Supplier profiles
3MMI: Medical Additive Manufacturing/3D Printing Annual Report 2018
Smith & Nephew Visionaire
- 4Based on internal Smith & Nephew sales data from 2017
- 7United Hospital Efficiency Program with VISIONAIRE Cutting Guides. St. Paul, MN.
- 8Based on published Smith & Nephew manufacturing protocols
5Mayo Clinic: First nationwide prevalence study of hip and knee arthroplasty shows 7.2 million Americans living with implants
93D Printing Industry: FDA Clears “First Ever” 3d Printed Spine Implant To Treat Of Multiple Injuries
103D Printing Industry: Lima Corporate’s Ebm 3d Printed Hip Plant ‘Could Last A Lifetime’




