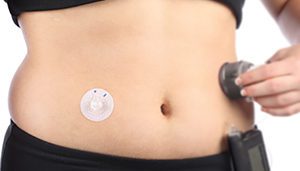
Too often the adhesive selection for a medical device is left to the end of a project. It doesn’t help that there are so many adhesives as to make the choice overwhelming. With so many potential negative outcomes, here are tips on making the right choice.
Tony Kaufman and Del R. Lawson, Critical and Chronic Care Solutions Division, 3M
If adhesive is an integral component in your medical device design, it needs to be thought about sooner rather than later. It’s often a factor left to consider at a point too late in the product development process – where changes in, say, device housing material or plans for final finishing processes would be too expensive or cumbersome to execute.Too often adhesive selection is approached with the mindset “tape is tape.” Perhaps different kinds might be interchangeable in everyday life, but that’s a dangerous oversimplification when designing a product someone will eventually be wearing and perhaps depending on to make critical health decisions. An adhesive’s characteristics and how it interacts with other device materials directly impacts the integrity and success of a device, regardless of whether the adhesive is adhering device components together or sticking the device directly to skin.