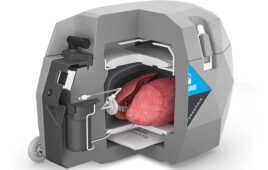
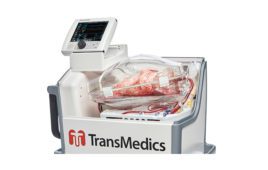
CytoSorbents (NSDQ:CTSO) and Aferetica today announced the European Union approval of Aferetica’s PerLife integrated ex-vivo system to perfuse, cleanse, recondition and preserve harvested kidneys and livers — including those that are usually discarded.
CytoSorbents (NSDQ:CTSO) and Aferetica today announced the European Union approval of Aferetica’s PerLife integrated ex-vivo system to perfuse, cleanse, recondition and preserve harvested kidneys and livers — including those that are usually discarded.
Italian startup Aferetica designed its PerLife system to improve organ function and viability while reducing the risk of primary graft failure and organ rejection. PerLife incorporates CytoSorbents’ PerSorb sorbent cartridge, which uses adsorptive porous polymer technology to reduce a broad range of toxins that can compromise organ function from the perfusate. Aferetica plans to launch the system in Italy this quarter.
One major targeted use is the reconditioning of unusable or sub-optimally functioning “marginal” organs that are typically discarded. This may increase the availability of scarce, suitable organs and improve the clinical outcomes of complex and expensive transplantation procedures, the companies have said. Today, this need is being driven by record rates of kidney and liver failure caused by globally pervasive health crises such as the aging population, diabetes, hypertension, obesity, alcoholism, hepatitis, and non-alcoholic steatohepatitis (NASH), also called “fatty liver.”
“The goal of the PerSorb cartridge is to remove harmful inflammatory toxins from the perfusion fluid and to restore a more conducive environment for organ health that may extend the length of storage and the quality of organ function at the time of transplant,” said Vincent Capponi, president and COO of CytoSorbents Monmouth Junction, N.J., in a news release. “The ability to provide high-quality organs capable of quickly regaining their function is expected to be an important factor in successful organ transplantation.”
“With the creation of PerLife and its EU certification, we are now entering the commercial phase for organ perfusion and real-world clinical usage. This follows five years of development and fine-tuning, including a fruitful three-year partnership with CytoSorbents to develop and integrate a new sorbent technology that can help to purify, clean, and preserve solid organs collected for transplant,” added Aferetica CEO Dr. Mauro Atti. “After an initial launch in Italy, we plan to market our therapy all over the European Union and in other countries that accept EU approval.”