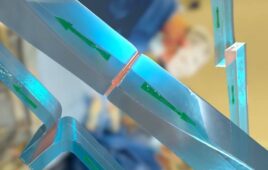
The hydrogel-based tough gel adhesive is formulated to be flexible, strong, and stays attached to biological tissues even when wet. (Image courtesy of Wyss Institute at Harvard University)
Harvard University’s Wyss Institute announced that it has licensed its tough gel adhesive technology to Amend Surgical, a Florida-based medical device company focused on developing and manufacturing novel biomaterials.
Amend Surgical has been granted exclusive rights to develop the technology for oral surgery applications. The company aims to redefine the current standard of care, which today often requires painful and irritating sutures to be placed by the clinician in the tight, difficult-to-access oral cavity.
The product is a biocompatible hydrogel-based material formulated to strongly bind to biological tissues, even in difficult, wet environments such as the oral cavity. After conducting initial validation and optimization of the tough adhesive, Amend Surgical plans to explore its use in products that release drugs or other active agents during wound closure, and in patches that promote dentin restoration.
The technology was created in the lab of Wyss core faculty member David Mooney and developed by postdoctoral fellow Benjamin Freedman and former postdoctoral fellow Jianyu Li.
“We are really enthusiastic about the application of our tough adhesive technology in dental surgery, as it has the potential to dramatically transform current clinical practice,” Freedman said in a news release.
“Both our engineering and clinical teams were involved in the evaluation of this important technology, and we are eager to begin commercialization,” added Amend Surgical CEO Robby Lane. “We envision that the tough adhesive could provide clinicians both a wound barrier and an effective means to protect bone grafts in a single, easy-to-apply product, and could potentially eliminate the need for sutures used in conjunction with most dental barriers.”
The company intends to ultimately manufacture the tough adhesive at its facility in Alachua, Fla., where it already employs a complementary manufacturing process.