The annual AORN Global Surgical Conference & Expo is renowned for having one of the largest, most bustling trade show floors in the healthcare field. Over 500 vendors will be on hand to tour their latest and greatest offerings.
With so many manufacturers and other providers in the exhibit hall, it can be difficult to navigate to make a firsthand connection with — or even steal a glance at — every exciting new product available.
Although it would be equally impossible to capture every transformational device here, these are some of the products new to AORN that we’ll definitely be seeking out.
Enthermics Titan Series Fluid Warmers and Combination Warmer

(Image credit: Enthermics)
This new line of patient warming systems is more affordable and allows medical professionals to purchase high-quality fluid warming cabinets. IV and irrigation fluids are safely warmed to recommended temperatures in these reliable warmers. All warmers are standard with glass doors for inventory management.
The Titan Series fluid warmers are available in 2.5 cubic feet and 3.5 cubic feet. The fluid warmers feature programmable controls with maximum temperature settings for injection fluids at 104°F and 150°F for irrigation fluids.
The EC1850bl is a dual-chambered warming cabinet with independent touch keypad controls. The upper fluid compartment holds up to 36 liters and can be set to safely warm either irrigation or injection fluids. The lower blanket chamber has a capacity of 30-40 blankets. This new warmer has a 20 percent larger capacity then leading warming cabinets with the same footprint.
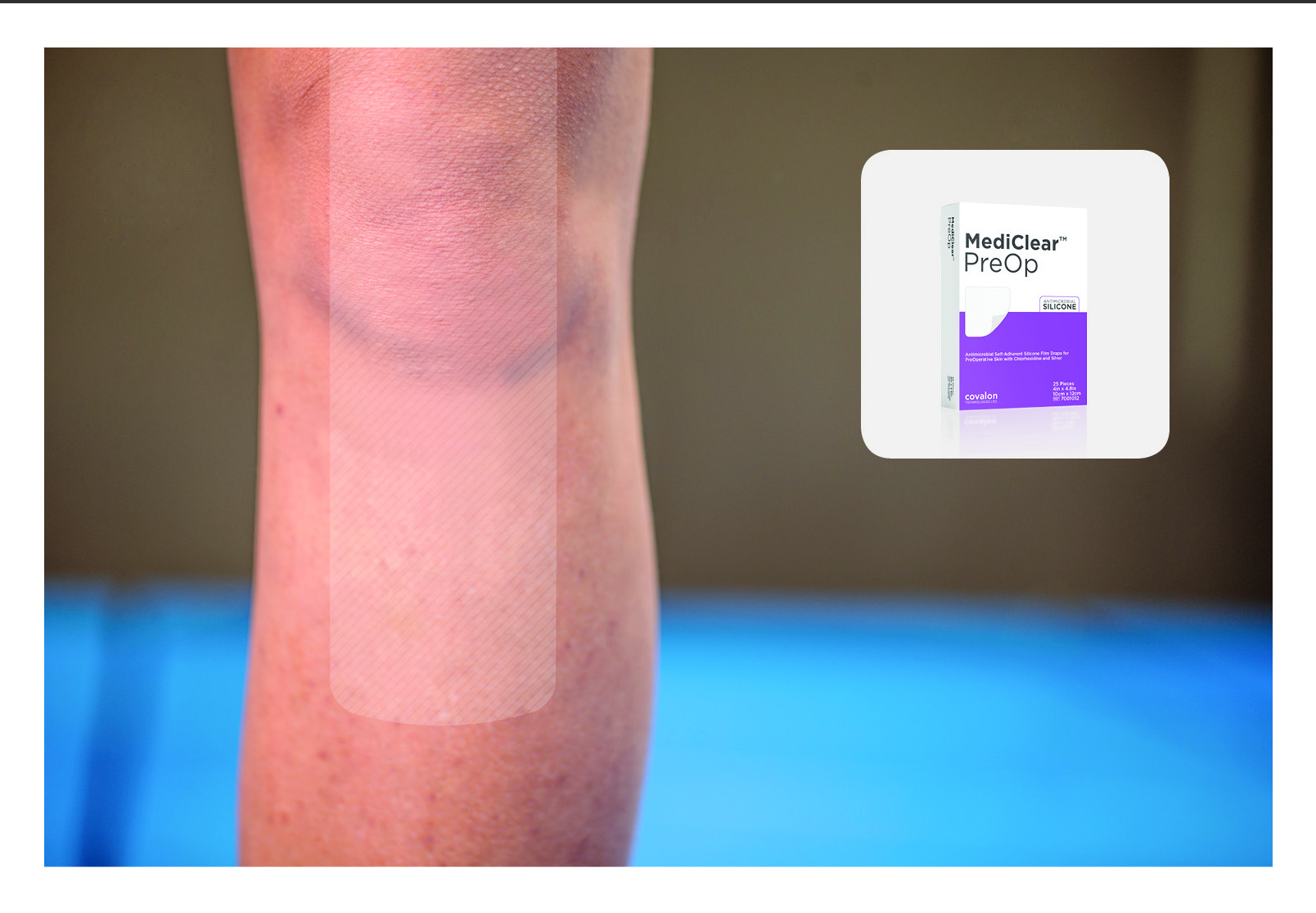
Covalon Technologies MediClear PreOp
(Image credit: Covalon)
MediClear PreOp is a self-adherent silicone film drape for pre-operative skin intended to cover and protect skin from the risk of contamination prior to an invasive procedure. Its breathable,
transparent, silicone adhesive contains chlorhexidine and silver for continuous antimicrobial activity with a rapid bactericidal and fungicidal effect against a broad-spectrum of microorganisms, averaging a 99.99 percent reduction at 30 minutes, and it prevents their re-growth for up to 7 days during wear.
The polyurethane barrier film acts as a protective patient covering to isolate a procedural site from microbial and other contamination.
MediClear PreOp has been cleared by the FDA for sale to U.S. hospitals and clinics and directly to patients without the need for a prescription.
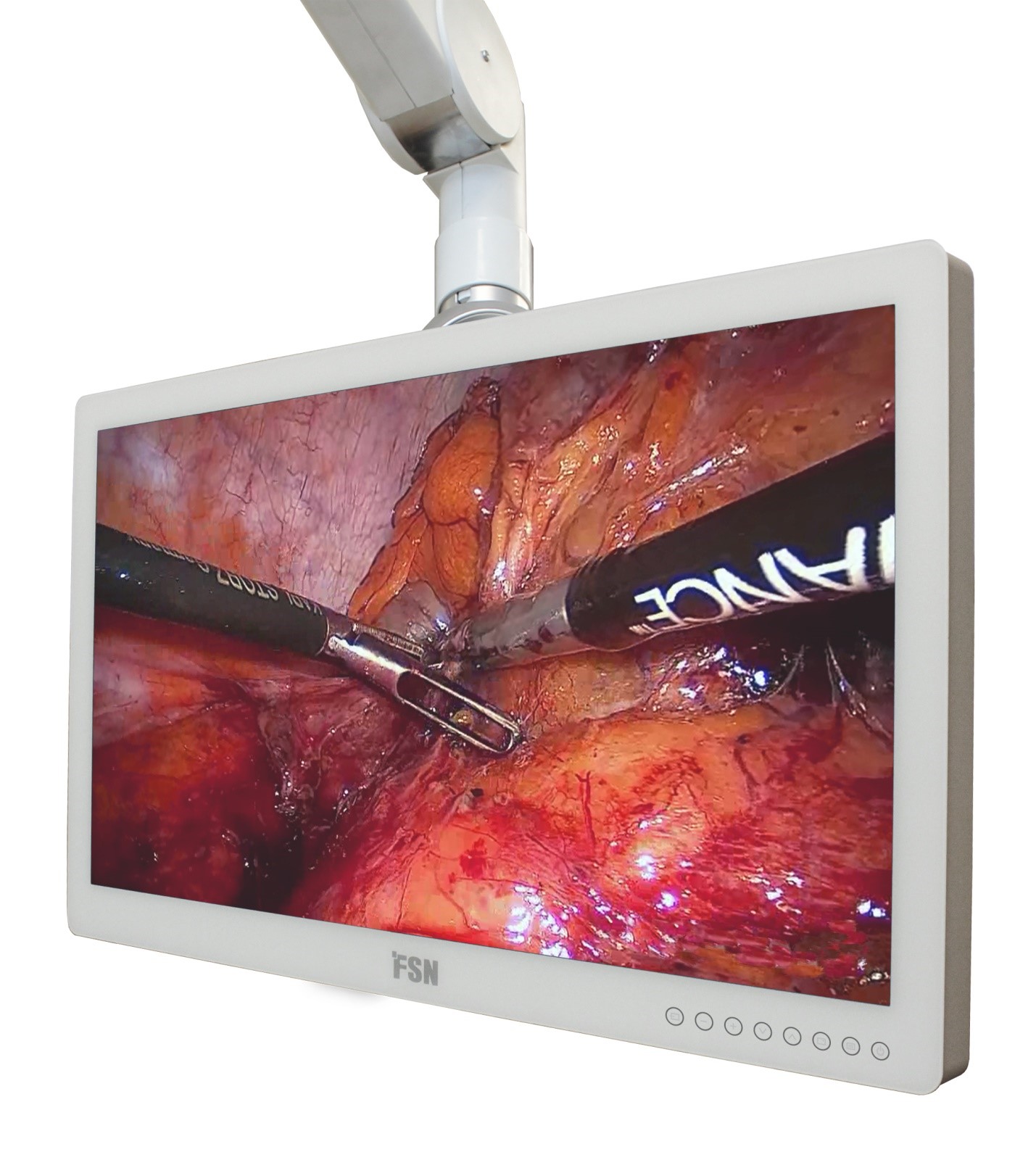
FSN FS-P2404D and FS-P2604D Monitors
(Image credit: FSN)
FSN engineers have been busy designing and developing the latest medical-grade display monitors, and the hard work has paid off. FSN is introducing a new 24 inch HD model, FS-P2404D, and a new 26 inch HD model, FS-P2604D.
The driving force behind these new products is the desire to continually provide the best in performance and value. Medical video applications will benefit from the features that have been designed into these display monitors.
New features found in FS-P2404D and FS-P2604D are impressive. A redesigned user interface menu is intuitive and self-explanatory. The front surface of these models is made from glass that extends completely from edge-to-edge, making sterilization practices much easier. The front filter is also bonded, a process that increases picture clarity, increases strength, and reduces susceptibility to humidity problems. A backlit menu area on the front bezel allows for gentle touch control.
Of course, FS-P2404D and FS-P2604D continue to provide the advantages and benefits of all FSN monitors. At their core they are made with high quality LCD panels that deliver top-tier image reproduction. This translates into reduced eye strain for medical professionals.
They are compatible with virtually all surgical video generating devices. With a large selection of signal inputs and auto-recognition capability, you can be sure that these display monitors from FSN will perform to the highest standards.
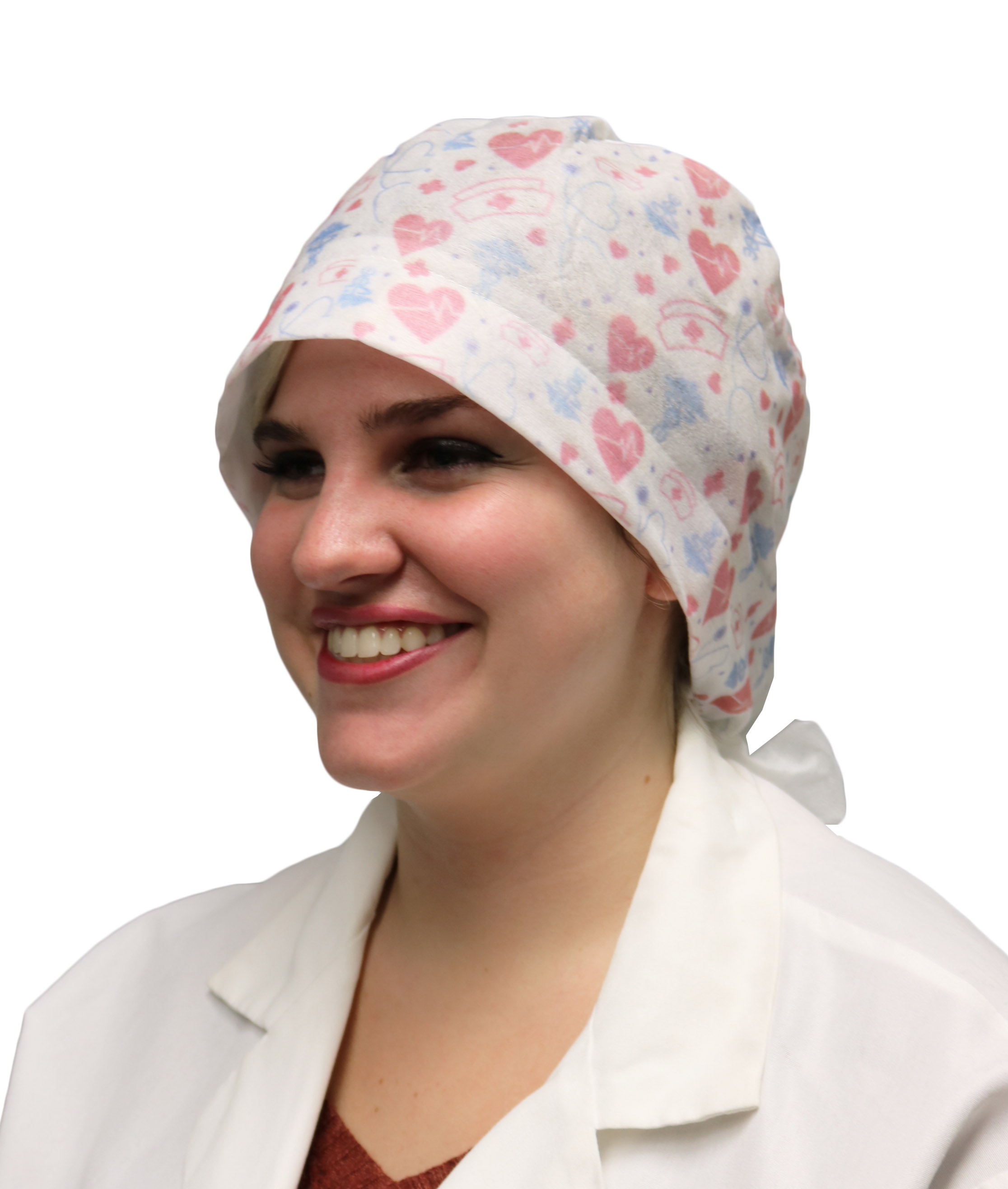
Healthmark Custom Headwear
(Image credit: Healthmark)
Custom-Printed Disposable Headwear is available in two styles: bouffant caps and scrub caps.
Disposable Bouffant Caps are manufactured from latex-free polypropylene fabric and have an elastic headband for a secure and comfortable fit. Disposable Scrub Caps are manufactured from spunlace nonwoven rayon material and include a tie-back closure.
Both styles are one size fits all and can be custom printed with the design of the wearer’s choice. Healthmark’s Custom-Printed headwear is intended to provide style and comfort to healthcare professionals while following industry guidelines.
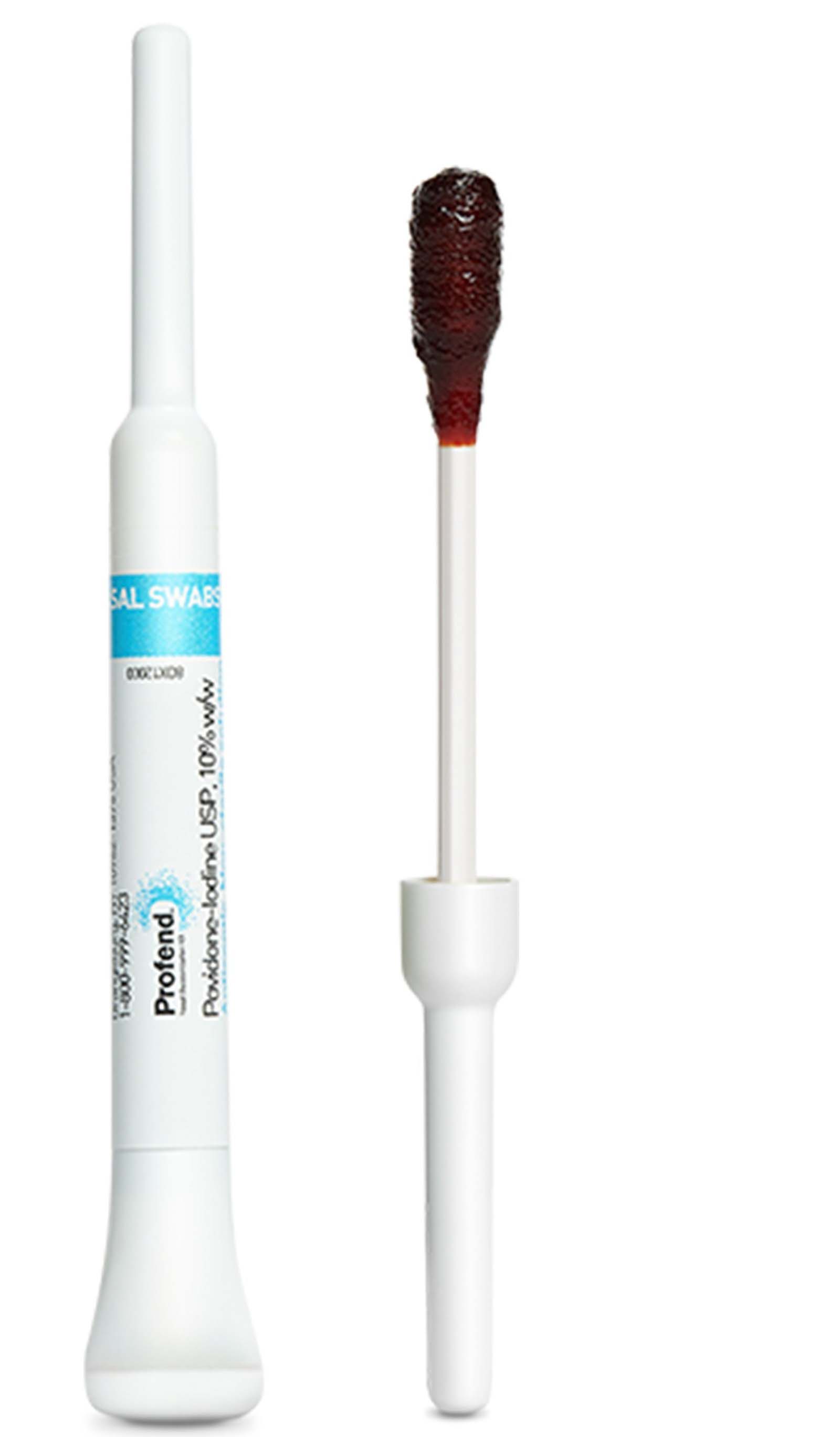
PDI Healthcare Profend Nasal Decolonization Kit
(Image credit: PDI)
Profend Nasal Decolonization Kit is the first in a suite of Profend products designed to help prevent surgical site infections (SSIs) and other hospital-acquired infections (HAIs).
Profend nasal swabsticks are pre-saturated with 10 percent Povidone-iodine to deliver a fast time to full efficacy against a leading risk factor for SSIs, S. aureus, and methicillin-resistant S. aureus (MRSA) in patient’s noses, without the risk of antibiotic resistance. At any given time, about 30 percent of the adult population is nasally colonized, according to a study from the Journal of Clinical Microbiology.
The Profend Nasal Decolonization Kit uses a unique “snap and swab” design that simplifies the decolonization process, while offering convenience for clinicians and supporting patient comfort.
Clinician-applied for assured treatment compliance, the swabstick’s dry-handle design minimizes mess during application, while the small nasal swab offers easy application and enhanced comfort for patients. Treatment time is a quick 60 seconds – up to two-and-a-half times faster than other nasal decolonization products on the market.
Additionally, Profend uses PVP-iodine, a broad-spectrum antiseptic proven effective against S. aureus and MRSA with no known pathogen resistance, as demonstrated by Houang et al. in the Journal of Clinical Pathology, thereby supporting antibiotic stewardship. In a study of healthy volunteers, Profend nasal swabsticks reduced S. aureus by 99.7 percent at one hour and 99.9 percent at 12 hours, according to a PDI in vivo clinical research.




