[Image from the American Heart Association]
Cycling on a stationary bike typically determines whether a person needs surgery. If the patient becomes breathless while cycling, it is typically recommended to have valve replacement therapy.
Gerry McCann, professor of cardiac imaging and honorary consultant cardiologist at the University of Leicester Department of Cardiovascular Science, conducted the research and found that the exercise test is “highly inaccurate” and results in thousands of patients having to undergo surgery when it isn’t needed and costs nearly £75M, according to a news release.
“Our findings showed that this exercise test, which has been approved by the American Heart Association/American College of Cardiology and the European Society of Cardiology, was highly inaccurate as almost twice the number of people who became breathless during the test did not develop symptoms within a year.”
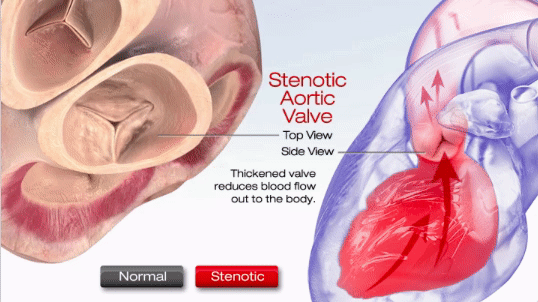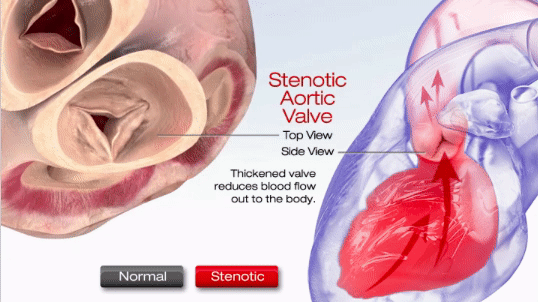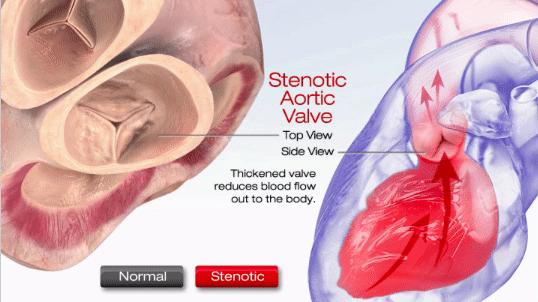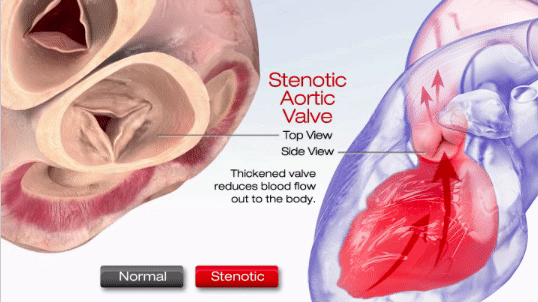
Aortic stenosis (AS) is one of the most common valve disease problems. It occurs when the aortic valve opening narrows and restricts blood flow from the left ventricle to the aorta and affects pressure in the left atrium, according to the American Heart Association. Symptoms include breathlessness, chest pain (angina), chest pressure, chest tightness, fainting, palpitations, the decline in activity level or heart murmur.
Approximately 10,000 aortic valve replacements are done a year and have a cost of up to £15,000 (equivalent to nearly $16,000). It takes anywhere between 7 and 10 days for a patient to recover after valve replacement surgery.
“There is no doubt that valve replacement therapy is highly effective for patients with symptoms, however, there are risks involved. It’s a major operation and there’s a 1% chance of people dying or having a stroke during or after. There’s also the chance they could develop an infection,” said McCann. “It can often take 6 months to recover, but if they survive they tend to do very well afterward. However, if we know a patient has AS and no symptoms and we do nothing there’s also a 1% chance they will die so there’s a fine line between whether we should intervene or not.”
McCann hopes to further develop this research to find more accurate ways for doctors to check symptoms for AS before performing surgery.
The study was published online in the European Heart Journal and was supported by an NIHR Fellowship.
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]




