Wearable devices are becoming more prevalent in the consumer environment with devices that monitor activity-related information, such as heart rate and caloric burn. Technical capabilities have allowed the use of wearable devices to make inroads into clinical applications, with devices for monitoring (such as glucose blood levels) and dispensing (insulin, drugs, electrolytes) coming to market. These devices are expected to become increasingly valuable, as they lessen the patient’s time in medical facilities and reduce healthcare costs.
The growing importance for these devices to be stable, safe for skin contact, and unobtrusive has increased the need for high performing adhesives for wearable device fixation. The adhesives used for wearable applications must perform consistently. An improperly affixed device is unlikely to achieve the desired wear time results when applied to the patient.
There is an ongoing need to develop more knowledge on the effects of adhesive/ substrate combinations and wear times. Evaluations can be performed on simple adhesive/substrate combinations without the backing of a wearable device, thus presenting a worst-case scenario in terms of material peeling off of the skin over time. Issues can then be addressed and corrected before a wearable goes to market.
Effective Human Wear Duration Studies
For the most meaningful results, it is best to use the same control adhesive/substrate combination throughout all studies. In the study explored here, the control was a nonwoven polyester fabric coated with 2 mil thick acrylic adhesive currently used to adhere a commercial insulin pump to the abdomen of patients.
This report covers four different enrollments of study participants; each enrollment is defined by the substrate that the adhesives were coated onto (Table 1). The substrates were chosen due to their commonality of use in the medical adhesive market, range of material composition, thickness, and Moisture Vapor Transition Rate (MVTR).
Five different proprietary acrylic adhesives (referred to by letters A-E) chosen for their previous use in skin-contact products were coated onto the substrates for this study (Table 2).
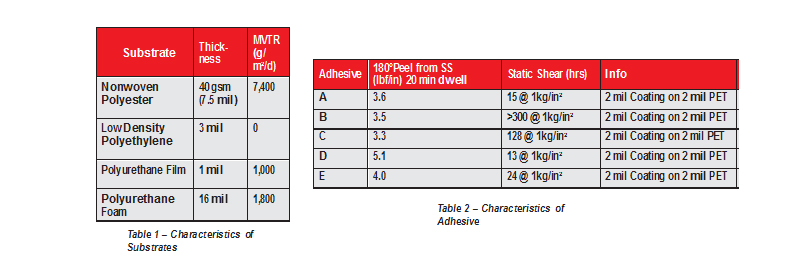
Table 1: Characteristics of Substrates and Table 2: Characteristics of Adhesives
Plan and Application
Ten participants were enrolled for each substrate phase of the study. Participants were screened to make sure that they did not possess any documented allergies or skin characteristics that could impact the study results. Participants were told to continue their daily activities, but avoid scrubbing the samples during bathing or exposing the samples to creams, moisturizers, and/or ointments.
Eight round (1 1/8 in./29 mm diameter) samples were placed on the inside arms of each participant, spread out so that two samples each were on the right lower, right upper, left lower, and left upper arms (Figure 1). With one control sample and four test adhesive samples, each study enrollment had five different sample constructions. These five sample constructions were distributed across the eight test sites per participant, such that each participant wore either one or two patches of each sample construction.
Sample placement across all participants was evenly distributed such that each sample variation was placed at each of the eight test site location on two participants, thus 16 of each sample variation was used in each study enrollment.
Every Monday, Wednesday, and Friday up to at least 18 days after affixing of the samples, the participants were questioned as to the loss of samples, along with the number of hours that the sample sites were exposed to sweat and bathed.
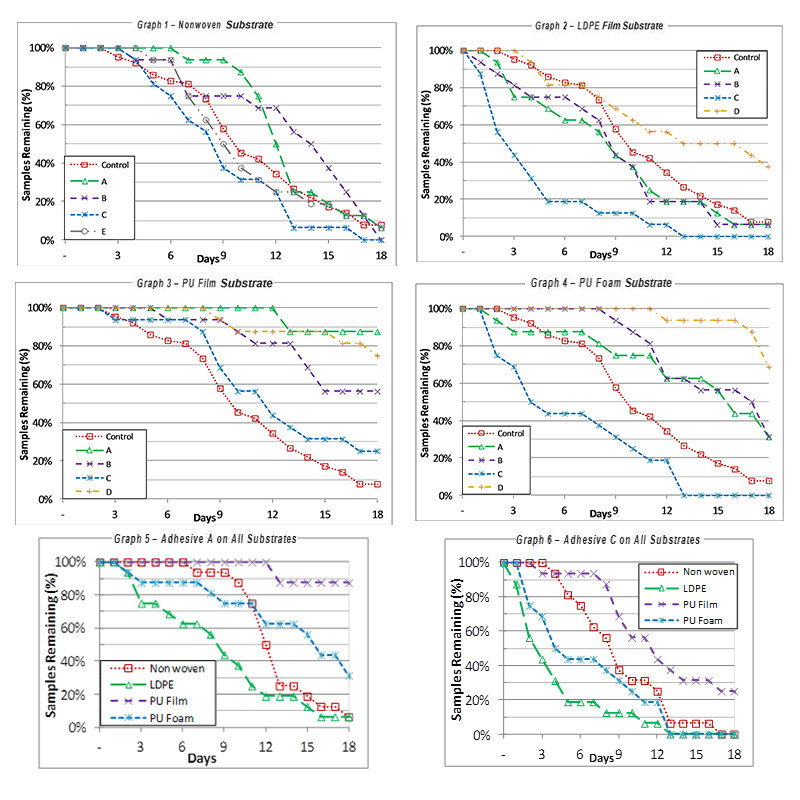
Graphs 1 through 6
Results
There was no significant difference observed in the effect of sample location, age, gender, hours of sweating or bathing on the wear duration of the samples in any of the studies. The graphs show the wear durations of different acrylic adhesives (A through E) on different substrates as percent of samples remaining on participants. After the nonwoven substrate study, Adhesive E, a medium performer, was replaced with adhesive D for the remaining three studies.
As can be seen, differences were observed in the wear duration for samples of the different adhesives on the same substrate (Graphs 1 through 4).
There was a wide range of wear durations observed depending on the acrylic adhesive/substrate combination. There was a tendency for Adhesive C to have the shortest wear duration on all substrates and for Adhesive A, B or D to have the longest adhesions depending on the substrate. The control, a nonwoven polyester, performed in the middle of the nonwovens in the upper realm, versus the LDPE samples, and toward the bottom of the PU film and foam samples.
The first sample could lose its adhesion (first failure) in as short as one day for some of the LDPE substrate samples, (Graph 2) and as long as 12 or 13 days for some of the PU film and PU foam samples (Graphs 3 and 4). There was also a wide range of wear duration for 80 percent of samples to be lost (a common wear criteria of interest) from < 2 days for adhesive C on LDPE to 17 days or greater for some of the PU film and PU foam samples. Table 3 shows the failure rates for the various substrates. Graphs 5 and 6 are comparisons of the wear duration of Adhesive A (Graph 5) and Adhesive C (Graph 6) on all four substrates.
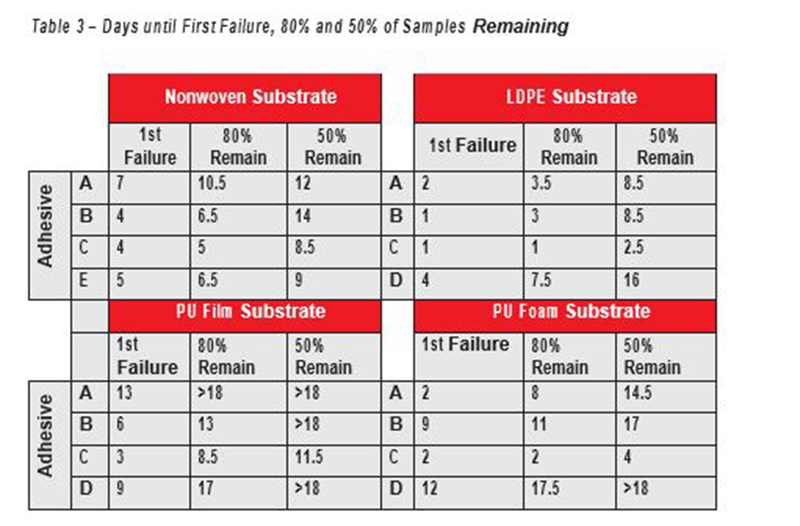
Table 3–Days until First Failure, 80% and 50% of Samples Remaining




