Avitus™ Orthopaedics Inc. (Avitus), a developer of medical devices that improve outcomes while decreasing healthcare costs for patients, surgeons, and hospitals, today announced that it has launched pilot sales of its Avitus™ Bone Harvester product.
The Avitus™ Bone Harvester, which has received 510(k) clearance from the FDA as a Class II medical device, is a novel surgical instrument for harvesting autologous bone graft and bone marrow in a minimally invasive manner for use in a range of spine and orthopaedic procedures.
“We are excited for the feedback and enthusiasm we have received from surgeons piloting our technology,” says Neil Shah, CEO of Avitus. “This validates the need for a streamlined way to obtain significant volumes of cancellous autograft and bone marrow. Autograft is regarded as the gold standard bone graft material; our technology has the potential to mitigate the harvesting challenges associated with autograft and provide a cost effective solution for surgeons using this bone graft option.”
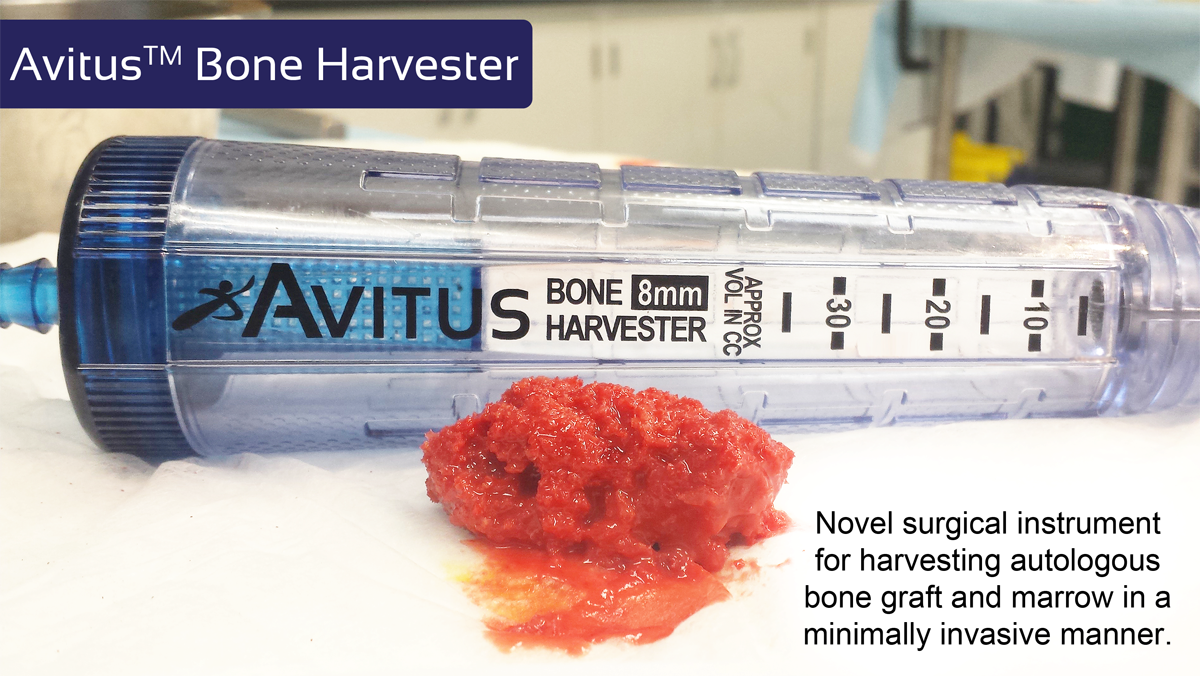
(Image credit: Avitus Orthopaedics)
Avitus expects to receive a CE mark for the Avitus™ Bone Harvester in Europe later this year. The company has begun to build partnerships in the U.S. and Europe with distributors who see the clinical and economic benefits of an autograft harvester as a strong strategic fit in their portfolios. Additionally, Avitus has been awarded a Small Business Innovation Research (SBIR) award from the National Science Foundation (NSF) to continue research and product development, allowing the company to expand its Avitus™ platform.
“Support from the National Science Foundation has enabled us to build an incredible team and has positioned us to expand our company’s product portfolio,” says Maxim Budyansky, Chief Technology Officer of Avitus. “We are developing a platform of products that will address a broader bone graft market with a focus on superior clinical efficacy and significant cost reductions when compared to current options. We are dedicated and excited to continue working with our key opinion leaders to innovate novel products that solve unmet clinical needs.”




