3. AliveCor: $150 million (Non-invasive monitoring)
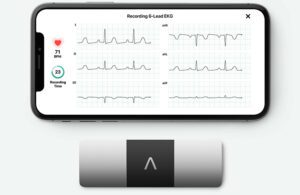
AliveCor’s KardiaMobile 6L measures heart rate and rhythm when a user presses their fingers to the device. [Image courtesy of AliveCor]
“This financing will help accelerate our growth into new strategic sectors and markets, enabling us to connect even more people to life-saving remote heart care,” AliveCor CEO Priya Abani said when announcing the funding round.
AliveCor’s FDA-cleared KardiaMobile 6L (6L stands for six lead) ECG detects heart rhythm conditions such as atrial fibrillation (AFIb).
The Mountainview, California-based device developer has been in the news for its patent fight with Apple.
AliveCor’s KardiaBand was the first FDA-cleared accessory for the Apple Watch in 2017. But the device maker ended KaridaBand sales in 2019 and sued Apple for patent infringement in 2020.
Last December, the International Trade Commission (ITC) ruled that the Apple Watch infringed AliveCor’s patented technology for AFib detection. President Joe Biden upheld the decision this February. (Note: A U.S. Patent Trial and Appeal Board decision favored Apple; AlivceCor is appealing.) .
“This decision goes beyond AliveCor and sends a clear message to innovators that the U.S. will protect patents to build and scale new technologies that benefit consumers,” Abani said at the time.
AliveCor hopes to take another bite out of Apple with an antitrust case expected to go to trial in 2024.