BIOTRONIK today announced the first patients enrolled in the BioInsight clinical study. The study evaluates the safety and feasibility of performing the minimally invasive BioMonitor 2 insertion procedure in an office setting.
BioMonitor 2 is an insertable cardiac remote monitor with ProMRI® technology that is placed underneath a patient’s skin to help physicians accurately detect and diagnose atrial fibrillation and syncope (fainting). Atrial fibrillation is a leading cause of stroke and heart failure. BioMonitor 2 provides the highest signal amplitude on the market, which leads to excellent sensitivity for improved reporting accuracy. The device can also be used to monitor atrial fibrillation in patients who have undergone ablation procedures. More than 2,000 BioMonitor 2 devices have been sold in the US since FDA approval in April 2016.
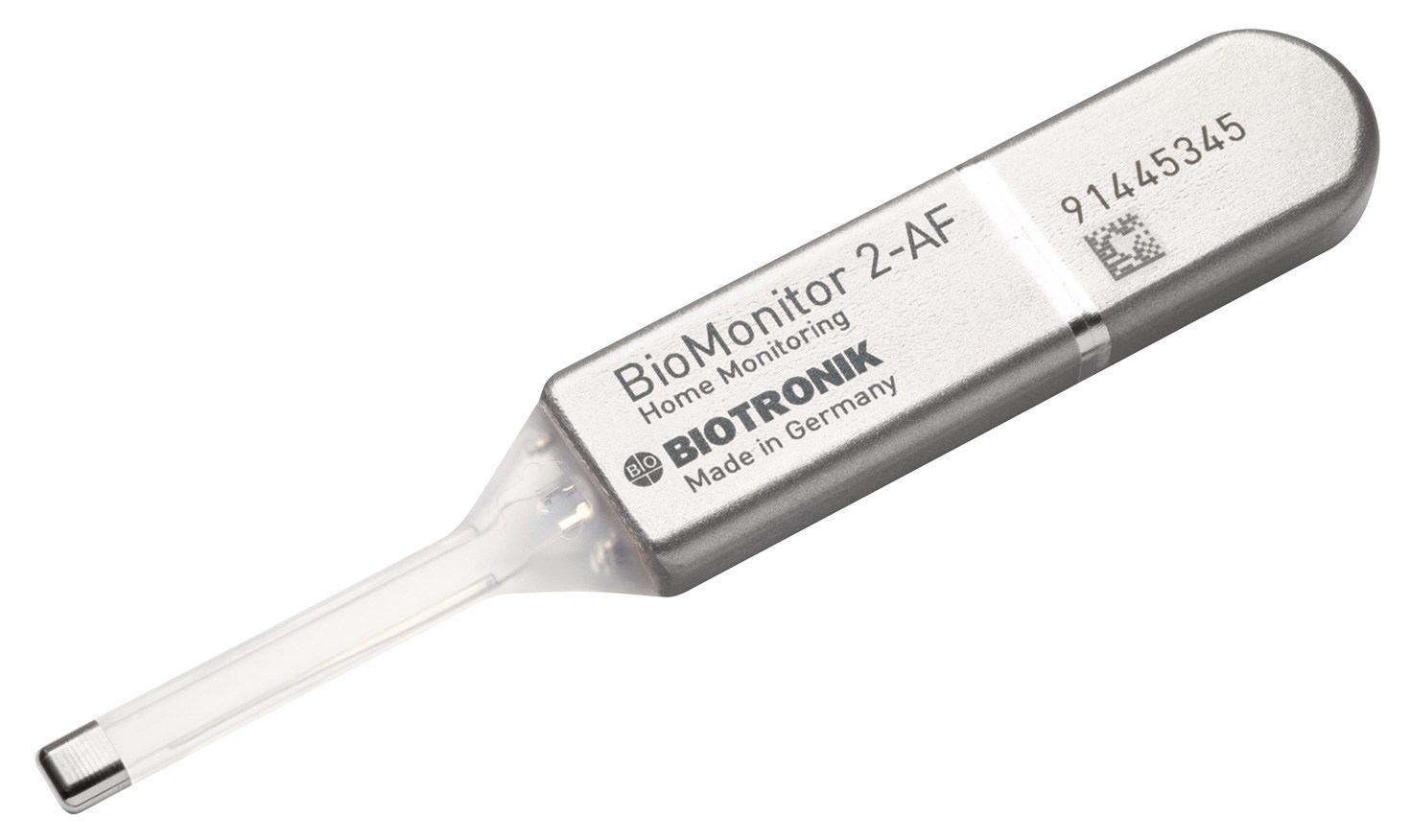
BioMonitor 2 is an insertable cardiac remote monitor with ProMRI(R) technology that is placed underneath a patient’s skin to help physicians accurately detect and diagnose atrial fibrillation and syncope (fainting). (Credit: PR Newswire)
“One of the benefits of BioMonitor 2 is the ease of the insertion procedure, which typically only takes a few minutes,” commented Dr. Raul Weiss, cardiologist at Ohio State University. “In-office procedures can reduce patient’s and physician’s time, increase access to the device and reduce cost burdens for healthcare systems. Early insertion will likely also reduce the time needed for a
conclusive diagnosis.”
The BioInsight study is a multi-center, prospective, non-randomized post-market study. Participants will receive BIOTRONIK’s BioMonitor 2 via in-office insertion and will be evaluated for 90 days to monitor for any potential adverse events, including infection and bleeding.
“BioMonitor 2 has been rapidly adopted by physicians, becoming a trusted, reliable solution to monitor for cardiac arrhythmias,” said Marlou Janssen, President, BIOTRONIK, Inc. “There is a significant need for BioMonitor 2, and we want to ensure our physicians have the utmost confidence in their ability to deliver efficient patient care. The BioInsight study will assure physicians and patients that performing the insertion procedure in an office setting safely and effectively improves access to this critical diagnostic tool.”
The BioInsight study is expected to be completed in the third quarter of 2017. Additional details about BioMonitor 2 are available on BIOTRONIK’s website.




