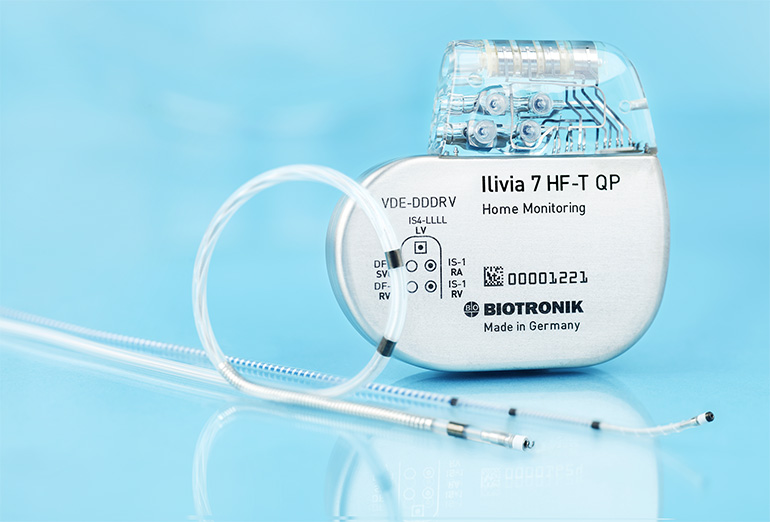
Biotronik has launched a new series of cardiac resynchronization therapy (CRT) devices with several functionalities that allow physicians to offer an all-inclusive and individualized solution for optimized CRT.
“Biotronik’s new CRT-Ds are equipped with numerous features that address physicians’ concerns about cardiac resynchronization therapy,” says Dr. Christof Kolb, German Heart Center, Munich, Germany. “The energy-efficient MultiPole Pacing has great potential to increase patients’ response to CRT and to sustain this in the long-term.”
(Image credit: Biotronik)
Biotronik’s Ilivia series of CRT-Ds is specifically designed to address the 30 to 40 percent non-response rate to CRT without causing disproportionate additional battery drain. The energy-efficient system features MultiPole Pacing (MPP) for sequential or simultaneous pacing in the left ventricle (LV) that is individually programmable for an increased response rate. To further improve therapy success, this is complemented by a diverse portfolio of LV leads for different patient anatomies and target veins, including a newly launched Sentus QP lead with alternative pole-spacing for short to medium veins.
Since CRT patients are likely to require an MRI scan during their healthcare journey, the Ilivia CRT-D offers ProMRI and is equipped with Biotronik’s award-winning MRI AutoDetect feature, which enables the device to automatically recognize an MRI environment within a programmable time window, and switch itself to and from MRI mode. This simplifies the process of MRI scans for both patients and physicians and decreases the duration of reduced therapy, offering increased patient safety.
“The launch of our latest generation of CRT-Ds and LV leads marks a significant expansion of our CRT portfolio,” says Manuel Ortega, senior vice president at Biotronik. “With a complete and innovative CRT solution, we want to empower physicians to offer a superior solution for heart patients that covers the entire duration of therapy and post-implantation care.”




