INTRODUCTION
Chronic Kidney Disease (CKD) is an increasing public health issue affecting more than eight percent of the global population. The most severe stage of CKD is End-Stage Renal Disease (ESRD) which is a total failure of the kidneys, requiring either dialysis or a kidney transplant for the patient to live. Statistics show that more than 50 percent of the patients suffering from ESRD do not meet the requirements for a transplant and hence depend on dialysis. An estimated 2 million people are currently receiving dialysis treatment worldwide. The majority of patients receiving this treatment are from five countries (US, Japan, Germany, Brazil and Italy) with a major populace of patients from the rest of the world not receiving treatment due to a lack of access to dialysis and the unaffordable cost of this expensive procedure(1).
Improved survival of patients on hemodialysis, coupled with the inability to provide enough renal transplants for the growing ESRD population, have resulted in an increase in the average time and number of patients on dialysis. When ESRD occurs, the kidneys cannot remove harmful substances from the blood. Hemodialysis removes the blood from the body and runs it through a special filter to eliminate the unwanted substances, and pumps the blood back into the body. The key requirement for hemodialysis is to draw the blood from inside the body. Access through a catheter is a short-term solution but longer-term, a connection between the arteries and the veins, known as an Arterio-Venous Fistulae (AVF) is established in the wrist or upper arm of the patients. When the AVF dilates, blood flow through it is increased substantially and this provides an access point to remove blood from the body for purification.
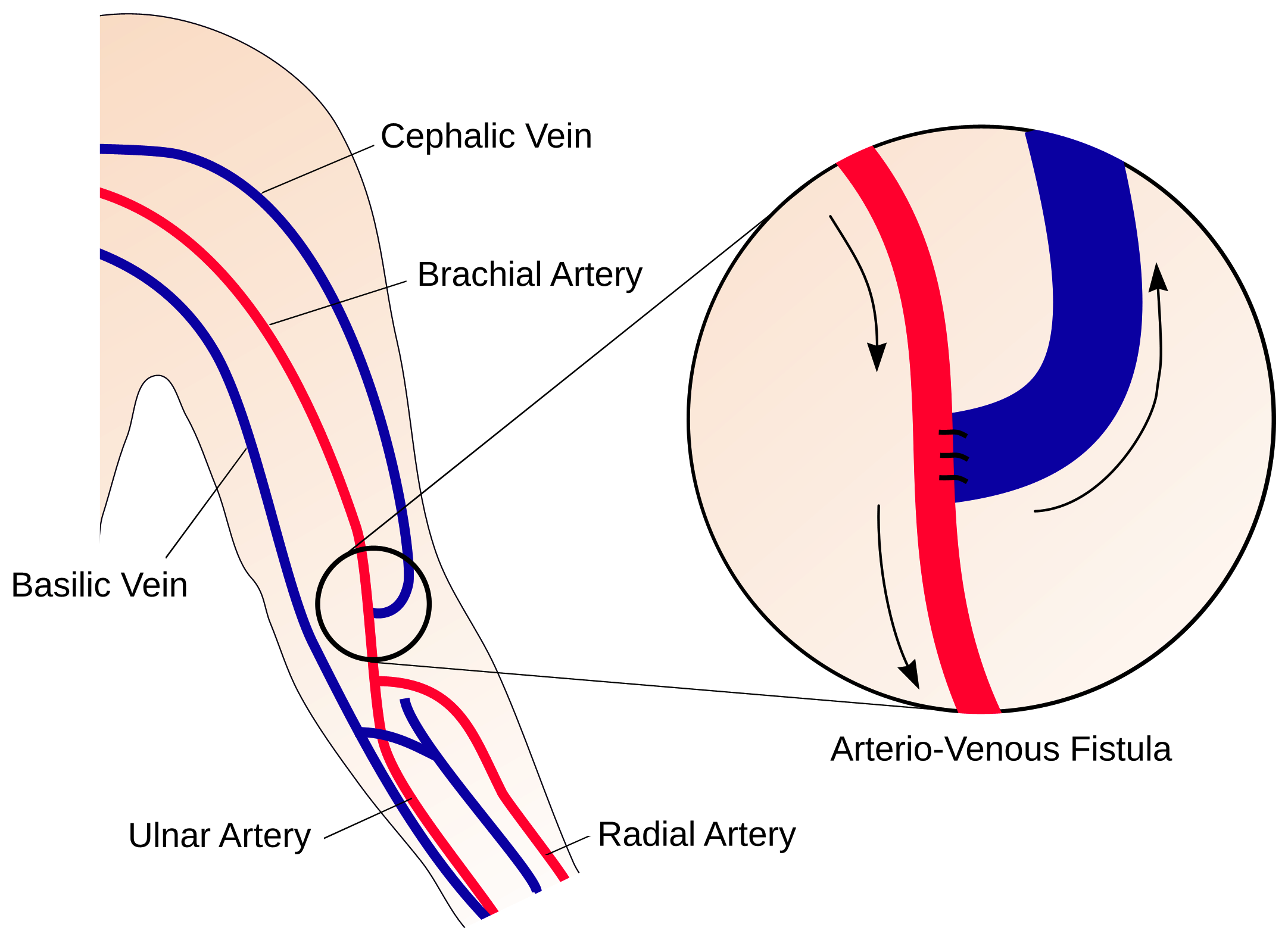
Figure 1: A schematic illustration of an AVF in the arm, formed by anastomosing a vein onto an artery. (Credit: Prof. Peter Vincent at Imperial College)
Complications associated with vascular access and in particular the stability of AVFs is a major cause of morbidity among ESRD patients [1]. The patency of AVF is often severely reduced by the inflammatory disease Intimal Hyperplasia (IH) and/or by thrombosis, causing unfavorable clinical outcomes, additional costs for healthcare systems and even death. AVF failures place a heavy cost burden on public-health systems, rendering such treatments expensive. These complications have established a need for a functional, durable and cost-effective vascular access.
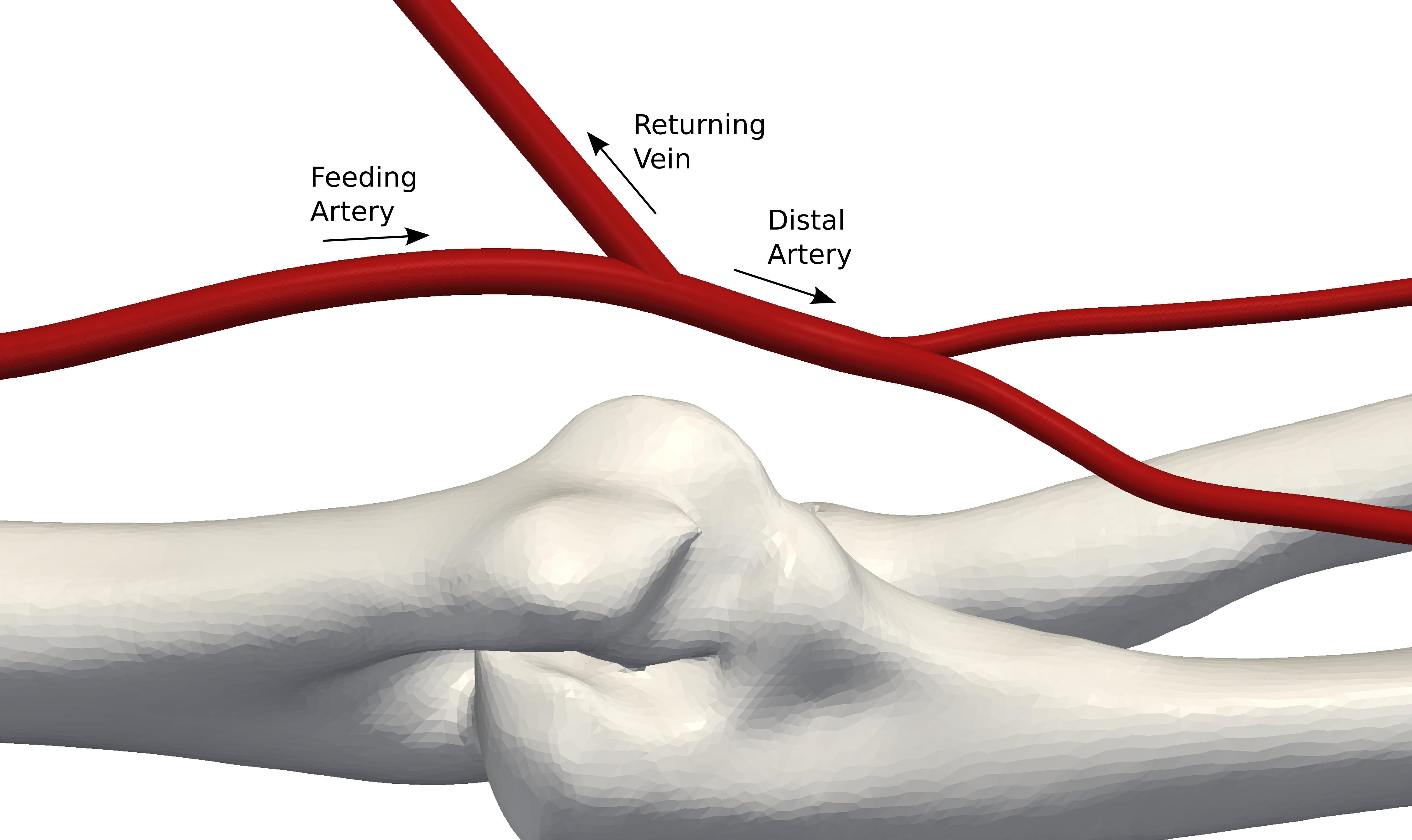
Figure 2: CAD model of AVF configuration formed in the arm via ‘virtual surgery’ (Credit: Prof. Peter Vincent at Imperial College)
 (1).png)
Figure 3: View of the volume mesh at the AVF (Credit: Prof. Peter Vincent at Imperial College)
A team of researchers from Imperial College London, are working towards using modern computational tools to develop novel AVF configurations with ‘favorable’ blood flow patterns, providing guidance to surgeons for dialysis treatments, and eventually making the procedure cheaper and less prone to failure due to IH. The team, consisting of researchers from Imperial College Renal and Transport Centre, the Dept. of Medicine, the Dept. of Bioengineering, and the Dept. Of Aeronautics, are collaborating with the Academic Health Science Centre and NIHR Comprehensive Biomedical Research Centre to use Computational Fluid Dynamics (CFD) to solve this internationally relevant healthcare problem. This article gives a brief overview of the research currently being undertaken at Imperial College London towards a safer, cost-effective dialysis procedure.
ARTERIO-VENOUS FISTULAE AND THEIR FAILURE
AVF are access points to the blood circulation for hemodialysis , created by a vascular surgeon using the patient’s native vessels. The vessels used – an artery and a vein -are joined (anastomosed) with the end of the vein attached to a 5mm hole made in the side of the artery [2]. The blood flow, diverted from the artery into the vein, causes the vein to become enlarged and thicker, allowing placement of a large-gauge needle. In fact, in response to the steep pressure gradient existing between the arterial and the vein, the blood flow rate will increase and eventually the access will be able to deliver a blood flow of 300 to 500 ml/min [3], necessary to perform dialysis. As a comparison, the normal blood flow through this area of the arm is around 50-100 ml/min.
Although AVF represents the gold standard of treatment for eligible patients, they still have a high failure rate of almost 50 percent [4] within the first month after their creation. Intimal Hyperplasia in the AVF occurs due to an abnormal thickening of the tunica intima of a blood vessel as a complication of the physiological remodelling process, triggered by altered flow conditions. This abnormal expansion negatively affects the patency of the AVF and eventually leads to its obstruction [5].
SAFER AVF DESIGN USING STAR-CCM+
In recent decades, CFD, a numerical simulation technology first developed for aerospace applications, has become a popular alternative to experiments and has been used as a design tool in the Life Sciences Industry. Applications of CFD include biomedical device design, numerical diagnostics and pharmaceutical manufacturing.
With the use of numerical simulation, the research team at Imperial College London analyzed multiple AVF configurations to understand the impact of the geometry on the blood flow patterns and the likelihood of failure. CFD allows the study of blood flow in the vasculature and any required metrics of the flow, to be calculated based on a definition of the vascular geometry and inflow boundary conditions. Experiments are often difficult to perform for obtaining flow patterns in AVF configurations and present various limitations in human subjects, resulting in only scant data being available. Metrics of most interest, such as Wall Shear Stress (WSS) and Oscillatory Shear Index (OSI), a measure of time variation of the direction of wall shear stress vector, are not readily available from experiments. Numerical simulation enables researchers to properly visualize such complex flow phenomena in greater detail and is non-invasive, with the ability to analyze multiple designs quickly and efficiently.
THE SIMULATION PROCESS
The process begins by obtaining a CAD model of the native arteries in the arm. Various AVF configurations are then formed on this native geometry via ‘virtual surgery’ (See Figure 2). STAR-CCM+, CD-adapco’s flagship software, is then used to simulate the blood flow through each AVF configuration. STAR-CCM+ is a single integrated package with a CAD-to-solution approach and optimization capabilities enabling the user to effectively analyze multiple design variants and optimize for best design.
The AVF configurations were discretized using the automated polyhedral cell meshing technology of STAR-CCM+ with approximately 10 million polyhedral cells for each design. A close-up view of a volume mesh with prismatic layers at the wall is seen in Fig 3. Automated prism layer generation on the walls was used to resolve the boundary layer flow at the wall of the vein, artery and AVF. The computational mesh was properly refined near the connection to capture the fine scale flow features. Incompressible Navier-Stokes equations were solved in the entire domain with the blood being modeled as a Newtonian fluid with constant viscosity. The inflow conditions for blood flow into the artery were considered to be non-pulsatile in the initial stage with further simulations incorporating transient pulsatile flow as the boundary condition. The walls of the vessel were considered as rigid, no-slip walls.
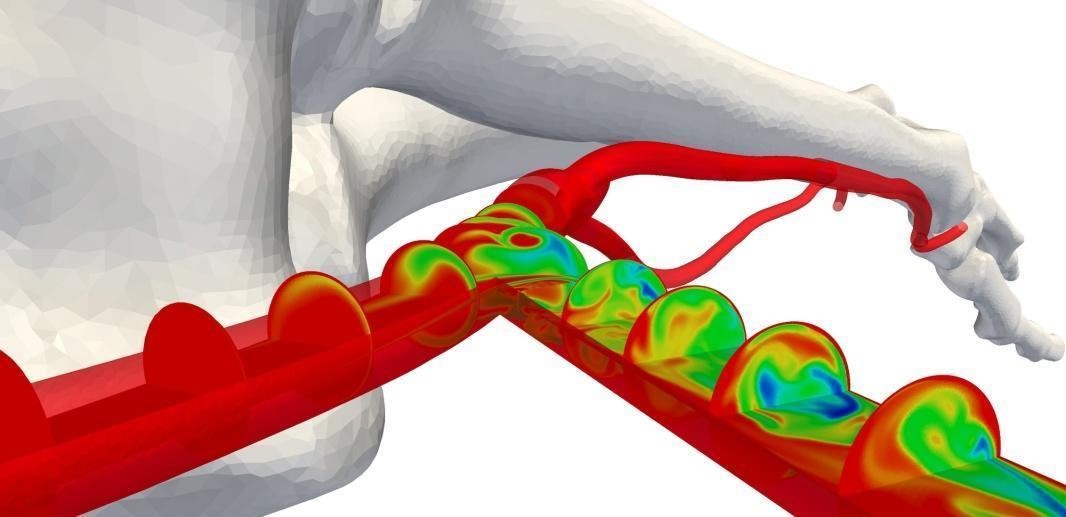
Fig 4: Concentration of a passive scalar, advected with blood flow, on different plane sections along the artery, vein and AVF (Credit: Prof. Peter Vincent at Imperial College)
Figure 4 shows the scalar transport of a passive scalar advected with the blood flow on planar sections at constant intervals along the artery, vein and the AVF connection for one of the designs. The concentration of the passive scalar gives a visual indication of how the blood is mixed in the AVF. The results from the simulation enable a qualitative assessment of the blood flow patterns.. The non-physiological hemodynamics in this region causes WSS to fluctuate greatly. This behavior could result in the failure of the AVF due to inflammation.
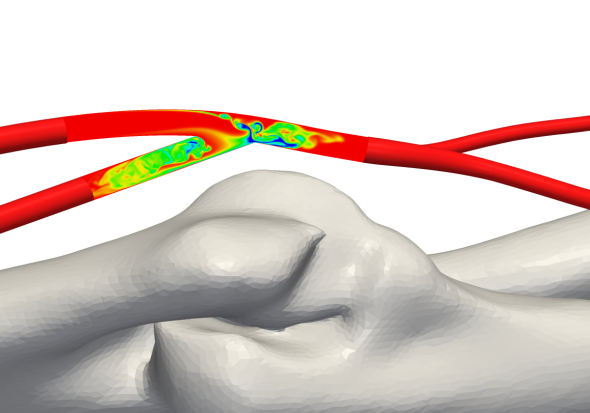
Fig 5: Concentration of a passive scalar, advected with blood flow, on a centerline plane section in the AVF (Credit: Prof. Peter Vincent at Imperial College)
CONCLUSION
The team of researchers from Imperial College London is undertaking numerical simulations to improve clinical outcomes for dialysis patients and reduce the financial burden for healthcare providers, by developing better designs to decrease the failure rates of AVF. Reduced rates of AVF failure will lead to improved patient experience, survival rate and cost-utility, making dialysis potentially affordable for lower-income populations. It is hoped that results from the study will also help solve a range of other long-standing healthcare problems, such as failure of vascular stents, arterial bypass grafts and organ transplants, due to IH. The ultimate objective of the research team is to provide guidance to the surgeons on the configuration of AVF for healthy flow patterns and reduced potential for failure. This serves as an excellent example of the far-reaching impact of numerical simulation into our daily life, helping save lives with the same ease as which it helps to build products.
REFERENCES:
[1] Feldman, H., Kobrin, S., Wasserstein, A. (1996). Hemodialysis vascular access morbidity, J. Am. Soc. Nephrol., 523–535, 1996. [2] Loth, F., Fischer, P. F., & Bassiouny, H. S., Blood Flow in End-to-Side Anastomoses, Annual Review of Fluid Mechanics, 40(1), 367–393, 2008. [3] Sivansesan, S., How, T.V., Black, R., Bakran, A., Flow patterns in the radiocephalic arteriovenous fistula: an in vitro study, Journal of Biomechanics, 32(9), 915–925, 1999. [4] Huijbregts, H. J. T., Bots, M. L., Wittens, C. H. a, Schrama, Y. C., Moll, F. L., Blankestijn, P. J. , Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative, Clinical journal of the American Society of Nephrology : CJASN, 3(3), 714–9, 2008. [5] Sivansesan S, How T.V., Bakran A., Sites of stenosis in AV fistulae for haemodialysis, Nephrol Dial Transplant 14: 118–120, 1999.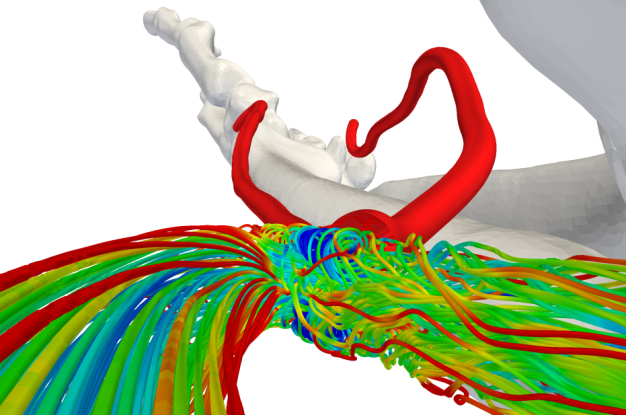
Fig 6: Streamlines showing flow features inside the AVF (Credit: Prof. Peter Vincent at Imperial College)


