Bluegrass Vascular Technologies recently announced positive results from a clinical study of its Surfacer Inside-Out catheter system.
The San Antonio-based company said the trial represents the first independent, multicenter study investigating the applicability of the Surfacer system, which is designed to provide access to the jugular vein to restore central venous access in hemodialysis patients with blocked neck veins. The trial’s results were published in the American Journal of Kidney Diseases.
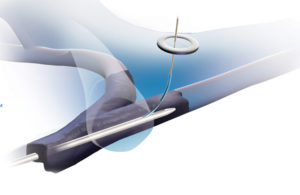
The Surfacer is designed to be threaded through the femoral vein up to and into blockages in the jugular, which acts as a stabilizer. The head of the catheter is then aligned via fluoroscopy with a target on the surface of the neck, and a needle guide is advanced through to the skin. A sheath is then inserted, the Surfacer retracted and a central venous catheter is attached to the sheath, according to the company.
The study enrolled 32 patients, of whom 83% had either Type 3 or 4 complex obstructions (three or more occluded vessels). Of those enrolled, 97% met both the primary and secondary efficacy endpoints of achieving central venous access through the use of the Surfacer system and catheter patency at three months, the company reported. No procedure-related complications were observed and no catheter-related infections occurred within the first week after catheter placement.
“Thoracic central venous occlusion is a significant issue, which can impact long-term morbidity, particularly in hemodialysis patients who depend on central venous catheters for dialysis therapy,” said Dr. Roman Reindl-Schwaighofer of the Division of Nephrology at Medical University Vienna, and first author on the study. “This study demonstrates that the Surfacer system is a reliable and safe means of repeatedly establishing vascular access, currently a challenge with alternative surgical options or sharp recanalization techniques.”
“The erosion of subclavian and left-sided vascular access routes are of great concern to this patient population due to their higher rates of central stenosis and catheter dysfunction,” added Dr. Vladimir Matoussevitch, M.D., vascular surgeon and head of the vascular access unit at the University Hospital of Cologne in Germany.
The Surfacer device won CE Mark approval in the European Union in August 2016; Bluegrass later inked an EU distribution deal with Merit Medical (NSDQ:MMSI) that included an equity stake.
“This study adds to the growing body of evidence highlighting the need for standardized treatment of central venous occlusions and demonstrating the positive clinical impact of the Surfacer System,” said Bluegrass Vascular president & CEO Gabriele Niederauer. “We look forward to broadening our clinical partnerships in Europe and the U.S. to best support this effort.”




