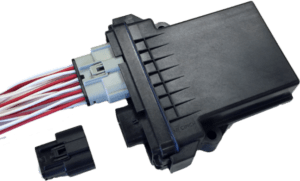
ModICE ME-MX is a line of environmentally-sealed connector enclosures for rugged control modules. The enclosures are available in various sizes along with various header/connector configurations.
Cinch Connectivity Solutions, a division of Bel Fuse, proudly announces the release of the ModICE ME-MX enclosure system. As an expansion of the ModICE product line, ME-MX adds USCAR compatible headers to the ME enclosure series.
ModICE is a line of environmentally-sealed connector enclosures for rugged control modules. The enclosures are available in various sizes along with various header/connector configurations.
ModICE is a turnkey solution for applications in agriculture, heavy equipment, commercial and recreational vehicles as well as general industrial controls. ModICE enclosures are rated IP67 and IP69K. Easy to assemble and the enclosures remain sealed even when the harness is not mated.
ModICE ME-MX offers a series of headers with SAE/USCAR-2 interfaces that mate with industry standard Molex MX150 harness connectors. ME-MX headers are available in 12, 20, 24, 32 and 40 I/O arrangements.
This product is ideal in applications such as:
- Vehicle Electronic Controls: CAN BUS, ISO BUS, Powertrain, Chassis and Hydraulics; and
- Telematics: Fleet management, Electronic Logging Device (ELD), Precision Agriculture and M2M.
Cinch Connectivity Solutions
cinch.com
Bel Fuse
belfuse.com




