Cleanrooms are an important production tool for life sciences manufacturers; maintaining high stands of product quality ensures product efficacy and builds a strong reputation.
Kevin Weist, president, Clean Air Products
Quality is paramount in the life sciences. The innovation and growth for which life sciences companies strive cannot be attained without top quality products.
Whether a company is involved in pharmaceuticals, medical devices or diagnostic equipment, lives often depend on the quality of finished goods. Impurities (microbial, chemical or other) in pharmaceuticals can trigger unwanted responses in patients. Those responses can be inconvenient, at a minimum, and life-threatening, at worst. Quality issues in medical devices pose a variety of risks to patients including life-threatening ones. No matter what the product, quality problems can damage a company’s reputation and even lead to expensive lawsuits.
A cleanroom – a room in which the concentration of airborne particles is controlled – is a critical manufacturing tool. It is used to minimize the introduction, generation, and retention of particles. (Other relevant parameters, such as temperature, humidity, and pressure, can also be controlled.) It helps a life sciences company manage the quality of finished goods and meet federal standards. Selecting the right cleanroom is crucial.
Cleanroom selection
When selecting a cleanroom, a manufacturer needs to consider:
- Particulate filtration.
- Design factors.
- Cost.
Filtration performance
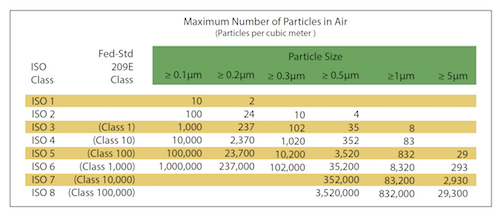
Government regulations, ISO guidelines, and internal requirements are the first considerations in selecting the right cleanroom. For example, ISO 14644-5:2004 guidelines specify basic requirements for cleanroom operations. These regulations and/or a company’s internal specifications will dictate the cleanliness level or required rating, a good starting point for choosing the right cleanroom.
Cleanroom design
Among the critical decisions a company has to make when adding a cleanroom are:
- Size
What processes should be included? What machinery or equipment is required and what amount of space does it take up? How many workers need to be accommodated?
The square footage requirements are needed in the design phase. - Performance
What level of particulate filtration is needed? (See chart above.)
This information is critical at the beginning of the process since it will dictate the number of filtration units needed and the type of filters used. - Siting
What is the most efficient location of the cleanroom?
A company will need to factor in overall workflow, ceiling height, floor condition, and accessibility. - Configuration
What will be the workflow in the cleanroom? How will materials enter/exit the cleanroom in a way that maintains particulate levels? How will workers enter/exit the cleanroom in a way that maintains particulate levels? Separate rooms and pass-throughs can be added for incoming materials and outgoing goods. An additional gowning room and air shower will reduce contamination from workers.
Cost
Cost is always an important consideration. Prices vary greatly from custom, fixed-wall construction to modular, free-standing, prefabricated cleanroom systems (soft wall or hard wall). Fixed-wall rooms are typically the most expensive choice, with soft wall, modular rooms the least expensive. Size, shape, configuration, and accessories will affect the overall cost.
Modular, free-standing cleanrooms reduce design, engineering and construction time. It can take several months to construct a fixed wall cleanroom because of up-front design and engineering requirements and the need to coordinate the various trades involved. On the other hand, a sophisticated modular room can be constructed in a week or two because all the modules are pre-engineered and manufactured. Onsite assembly is all that is required.
Since they are not an integral part of a larger structure, modular rooms can be taken down, reconfigured, moved or even sold. Expanding or reconfiguring a modular cleanroom can be accomplished by simply adding or moving modules.
Cleanroom use
Cleanrooms are not error-proof. To get the most out of an investment in a cleanroom, workers must be educated. Also, once installed, the cleanroom’s performance needs to be monitored, and there may be strict maintenance and cleaning protocols to follow.
Worker education
Manufacturers who make the investment in a cleanroom need to ensure that workers use the facility appropriately. Education on proper cleanroom procedures can be a requirement for access to the inside of the cleanroom. This training can maintain a high standard of cleanliness within the cleanroom and emphasize the seriousness of quality control. Having workers who are specialized and educated for the cleanroom environment reduces the chance of worker error.
Education for cleanroom workers should cover:
- Gowning
- Hand hygiene
- Use of gloves
- Specific job-related procedures
- Use of pass-throughs and air showers
- Wiping down work surfaces
Monitoring
Real time monitoring with cameras will record processes, record breakdowns for review and facilitate continuous improvement. By equipping a cleanroom facility with video cameras, a manufacturer can pinpoint errors and make adjustments going forward.
Real-time particle counting demonstrates a manufacturer’s willingness to meet a high level of quality. Cleanrooms can be equipped with sensors to count the level of airborne particulate matter at certain points in the manufacturing and assembly process. These sensors, taking measurements regularly and frequently, can be connected to a monitoring system that activates a stoppage sequence if a threshold for airborne particulates is crossed.
Maintenance and cleaning
Regular cleanroom maintenance is needed to ensure ongoing performance. At minimum floors and work surfaces are wiped down at the beginning and end of each shift. Other recommended cleaning procedures will be unique to the cleanroom and the manufacturing environment.
Life sciences manufacturing environments are especially exacting. Special cleaning procedures may be required using cleaning products that are cleanroom-approved. That includes detergents for removing dirt and grease – and disinfectants for controlling microbial contamination. Disinfectants have differing efficacies, defined as follows:.
- Bacteriostatic is used to halt bacterial growth.
- Bactericidal destroys bacterial cells.
- Sporicidal destroys endospore forming bacteria.
A life sciences manufacturing environment may need to use at least two disinfectants with different modes of activity to ensure thorough anti-microbial cleaning.
HEPA filters have a pre-filter that needs regular replacement. The HEPA filters themselves are maintenance free, but are required to be certified every year. Also, proper air flow and leak checks are part of the regular certification of a cleanroom.
Certification can be performed by either internal personnel or external companies. Most companies prefer an external, third-party certification because it provides independent analysis. Product requirements may require independent certification.
In conclusion
A cleanroom is an important production tool in a life sciences manufacturing setting. Control of particulate contamination (including microbial contamination), is essential to product quality. Maintaining the highest possible product quality will ensure product efficacy and build a strong reputation. Maintaining top quality also means a reduction of rejected products which saves production dollars.
When reviewing cleanroom options, a life sciences company should consider particulate filtration performance, design needs, costs, and maintaining the performance of a cleanroom through activities such as worker education, real-time monitoring, and maintenance and cleaning.
Kevin Weist is president of Clean Air Products (Brooklyn Park, Minn.).