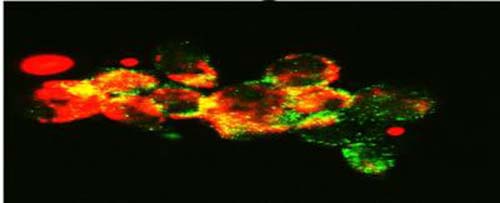
This image shows confocal microscopy detecting mitochondria (Tom20, red) colocalization with autophagosomes (LC3B, green), a process that happens during mitophagy in cancer cells treated with FLT3 inhibitor. (Credit: Image courtesy of Dr. Besim Ogretmen and Mohammed Dany of the Medical University of South Carolina)
Researchers at the Medical University of South Carolina Hollings Cancer Center have discovered a mechanism that confers resistance to drugs used to treat certain types of acute myeloid leukemia (AML). Targeting this pathway with a novel lipid-based therapeutic showed efficacy in a preclinical model of AML. These findings were reported in an article published in the October 13, 2016 issue of Blood.
“There are not many successful therapeutics at the moment for the treatment of patients with AML due to the problem of drug resistance,” said Besim Ogretmen, Ph.D., SmartStateTM Endowed Chair in Lipidomics and Drug Discovery at the Medical University of South Carolina (MUSC) Hollings Cancer Center and the senior author on the article.
In the Blood article, Ogretmen and his colleagues, including clinicians at the MD Anderson Cancer Center who provided patient samples, report that ceramide-dependent mitophagy plays a key role in chemotherapeutic-mediated AML cell death.
“Ceramide, a pro-cell death lipid, kills cancer cells by causing them to eat their own mitochondria,” said Ogretmen. “This is called mitophagy.”
Patient cells with the FLT3 mutation inhibit ceramide synthesis and thereby become resistant to cell death. To combat this resistance, a number of FLT3 inhibitors have been developed and trialed in patients with AML.
“Unfortunately, regardless of the inhibitor, the problem of resistance to FLT3 targeted therapy has persisted,” said Mohammed Dany, MD/PhD student and the first author on the article.
By adding a synthetic ceramide analogue, LCL-461, the researchers were able to reactivate mitophagy and kill drug-resistant AML cells in a dish. Mice with drug-resistant human AML tumor xenografts–that is, mice into which drug-resistant tumor cells from AML patients had been grafted-were also treated with LCL-461. The treatment eliminated AML cells from the mice’s bone marrow.
LCL-461 has clinical appeal because it is able to specifically target cancer cells. A positively charged molecule, LCL-461 is attracted to the mitochondria of cancer cells, which become negatively charged through the “Warburg effect.” This limits off-target effects that can occur with less specific inhibitors of FLT3 signaling. Previous studies in Ogretmen’s laboratory have tested the safety of LCL-461, finding that it had no major side effects at therapeutically active doses.
These results suggest the promise of LCL-461 as a potential therapeutic for patients with FLT3 mutated AML. LCL-461 was developed at MUSC in the Lipidomics Core. The MUSC Foundation for Research Development, MUSC’s technology transfer enterprise, has patented it and licensed it to Charleston-based startup SphingoGene, Inc.
Ogretmen and his colleagues are next seeking to perform large animal studies with LCL-461 to achieve Investigational New Drug (IND) approval, poising LCL-461 for translation.
“We are very excited about this. Head and neck cancers also respond to this drug very well,” said Ogretmen. “What we are trying to do is really cure cancer one disease at a time, and we are digging and digging to understand the mechanisms of how these cancer cells escape therapeutic interventions so that we can find mechanism-based therapeutics to have more tools for treatment.”




