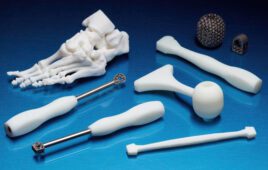
Stratasys is looking to take medical modeling to another level with its new J750 Digital Anatomy 3D printer. [Image courtesy of Stratasys]
Stratasys officials think the new printer will reduce the need to use human cadavers and animals for medical device testing and validation. There could also be a market to assist medical training and surgical preparation at academic health centers.
“We’re giving surgeons a more realistic training environment in no-risk settings. We also anticipate this will enable medical device makers to improve how they bring products to market by performing design verification, validation, usability studies and failure analysis with these new models,” Eyal Miller, Stratasys’ healthcare business unit head, said in a news release.
The J750 includes an updated version of Stratasys’ GrabCAD software — called GrabCAD Digital Anatomy — that includes a slicer to precisely control the properties of the various materials used to make a model in order to better mimic various tissues, Scott Drikakis, head of medical at Stratasys, told Medical Design & Outsourcing. Drikakis said Stratasys is focusing heavily on having third parties such as academic health centers and medical device companies validate anatomical information and publish it in order to further build out software data.
Drikakis spoke of the software enabling the design of “models based off what you’d expect to see in the native tissue or bone.” Users can define colors, transparencies, textures and finishes, and more.
“We hope everyone can continue to build on it,” Drikakis said.
The J750 3D printer comes also with new materials: TissueMatrix, GelMatrix and BoneMatrix. The materials are meant to support cardiac, vascular and orthopedic modeling applications. Drikakis boasted that the TissueMatrix — at 00 Shore A — will be the softest 3D printing material on the market.
Stratasys is also releasing a Blood Vessel Cleaning Station to remove support material from inside 3D-printed blood vessel models. Stratasys officials declined to disclose an exact price but said the J750’s pricing will be similar to other printers in the company’s J series.
Drikakis spoke of close collaboration with medical device companies and health providers to create the J750 — as well as pioneering researchers such as Michael McAlpine at the University of Minnesota. “We’ve had hundreds of surgeons practice on this material, give us feedback, ‘This is accurate. This is not. This feels similar.’ It was a collaboration of the medical community to get it to this point. We are not a medical device company, nor are we a hospital. So who better to provide the feedback than the people doing this every day.”