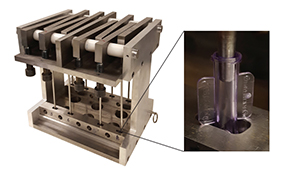
Covestro developed a new testing apparatus to respond to the needs of greater chemical resistance in luers. [Photo courtesy of Covestro]
Cancer treatments are hard on plastics, noted Lauren Zetts, North America market manager for medical and consumer products – polycarbonates at Covestro (Leverkusen, Germany). She said the aggressive solvents in oncology treatments such as benzyl alcohol and dimethylacetamide cause cracks in IV connectors. In 2015, FDA and the Institute for Safe Medication Practices issued a warning to health practitioners to avoid using closed system transfer devices with polycarbonate because of the risk of leakage and breaking.
Another challenge is that the connectors rely on a tight fit over time to prevent leakage during use. Polycarbonate is often chosen because it is less likely to loosen over time or at elevated temperatures due to stress relaxation.
Makrolon Rx3440 was developed to meet the high requirements of IV connectors. It has biocompatibility and toughness to resist mishandling. The material is also designed to allow thin-wall designs.
To test for both chemical resistance and stress relaxation resistance, Covestro developed a new apparatus that emphasizes immersion under real-world loading. It has an adjustable force to allow accelerated testing. Zetts noted that Covestro customers also are invited can test their existing luer connectors on the apparatus as well. She said the testing is comprehensive and meets the demands of tougher real-life situations found in oncology.
Using the new apparatus, Covestro has been able to show that compared with other transparent resins used for IV access components, Makrolon Rx3440 polycarbonate provides better chemical and oncology drug resistance. This material also demonstrates the best retention of stress to provide more reliable IV connections.