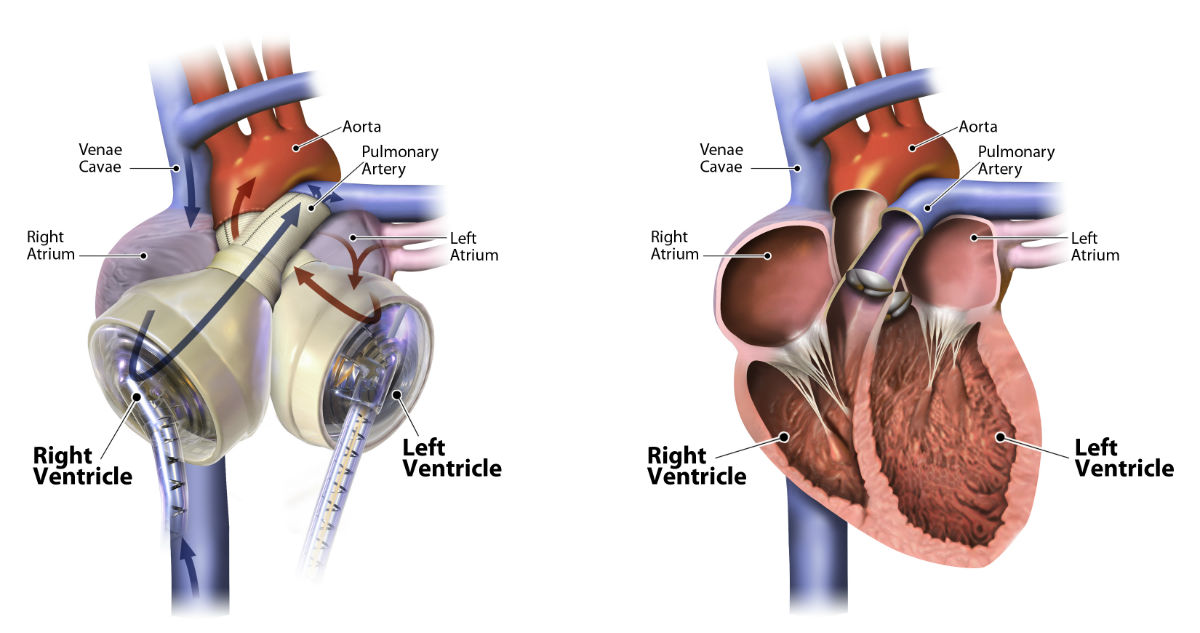
The SynCardia Total Artificial Heart, at left, and a human heart, at right. (Credit: SynCardia)
Dr. Mario Deng is a professor of medicine and medical director of the Advanced Heart Failure, Mechanical Support and Heart Transplant program at UCLA. This essay was published Sept. 4 in Live Science.
When Nemah Kahala, a wife and mother of five, arrived at Ronald Reagan UCLA Medical Center as one of our patients in March, her condition was the definition of critical.
She suffered from inflammatory restrictive heart muscle disease, in which the heart ventricles become stiff and rigid, making it impossible for these chambers to relax normally and fill with blood. Over time, this reduces blood flow and can lead to heart failure. Her condition was so advanced that repair surgery couldn’t help. It was clear she wouldn’t survive the wait for a heart transplant.
To buy some time, my colleagues and I wanted to replace Kahala’s failing heart with an artificial organ that would offer mechanical support until a donor heart was found. The artificial heart would provide an immediate and safe flow of blood to help her vital organs recover and make her a better candidate for transplant.
But there was another problem: Kahala, a petite 44-year-old, had a chest cavity too small for the artificial heart approved as a bridge for transplant by the U.S. Food and Drug Administration (FDA) in 2004. That version fits a majority of men and some women, designed for use in patients with a body surface area of 1.7 square meters (18 square feet). The device, meant to help those with life-threatening, nonreversible biventricular heart failure, has benefitted more than 1,440 patients so far. But it was too big to fit Kahala.
However, there was one more option. There is a smaller, investigational version of the larger heart, known as the 50cc SynCardia temporary Total Artificial Heart, made for use in a majority of smaller women and men, as well as many adolescents, with end-stage biventricular heart failure. In this condition, both sides of the heart fail to pump enough blood to sustain the body. Under a one-time emergency use permitted by FDA guidelines, Kahala became a candidate to receive the heart.
An unapproved medical device can typically be used only through a clinical study in which participants meet specific criteria and the device is only used in accordance with the trial. However in some cases, a doctor may request to use an unapproved device to save a patient’s life or to help a patient suffering from a serious condition for which there are no other alternatives. Due to the nature of Kahala’s case, she was eligible to receive the investigational, smaller heart under FDA guidelines.
A Second Chance
Much like a heart transplant, the artificial device replaces the heart’s two ventricles and four valves, eliminating the cause of end-stage biventricular failure. The device instantly provides a high volume of safe blood-flow to assist vital organs in recovering faster and making patients better candidates for transplants. Beforehand, Kahala was placed on a life-support system, but this remedy would have only worked for about 10 days before her organs would begin to deteriorate. Her condition was worsening so rapidly that we knew her only option was a heart transplant.
The two-hour surgery to implant the smaller, artificial heart was successful, and while hospitalized in the intensive care unit, Kahala began daily physical therapy to strengthen her for transplant surgery.
Three weeks after receiving the artificial heart, Kahala got her new donor heart. She is the first person in California to receive the smaller Total Artificial Heart, and the first patient in the world with the device to be bridged to a successful heart transplant — going from needing a transplant to receiving one.
Since 2012, the UCLA Heart Transplant Program has implanted eight 70cc artificial hearts. UCLA also participated in the clinical study of a 13.5-lb. (6.2 kilograms) Freedom portable driver — a backpack-sized device that powers the artificial heart, allowing the patient to leave the hospital — that received FDA approval last year. This device greatly benefits patients because it allows them to live at home and undergo rehabilitation, improving their condition and quality of life as they wait for their transplants.
The FDA cautions that the 50cc artificial heart is an investigational device, limited by U.S. law to investigational use. It is in an FDA-approved clinical study, in which UCLA plans to enroll patients soon.
The smaller artificial heart has large implications. It will allow doctors to expand the population of advanced-heart-failure patients who have biventricular heart failure and help patients who are smaller, like younger recipients and petite adults.
It’s evident that because of her condition, Kahala would not have survived while waiting for a transplant, and she’s not alone. Heart failure is a major issue in the United States, affecting nearly 5.7 million Americans, including 2.5 million women. This experimental technology saved Kahala’s life, and has the potential to help many other patients like her in the future, regardless of their shape and size.




