DePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, announces the U.S. launch of its Femoral Recon Nail System, a nailing system that provides new options for the surgical treatment of a wide variety of long thigh bone fractures. This new system builds on the company’s comprehensive trauma portfolio of innovative solutions designed to improve outcomes for patients with hip and femur fractures.
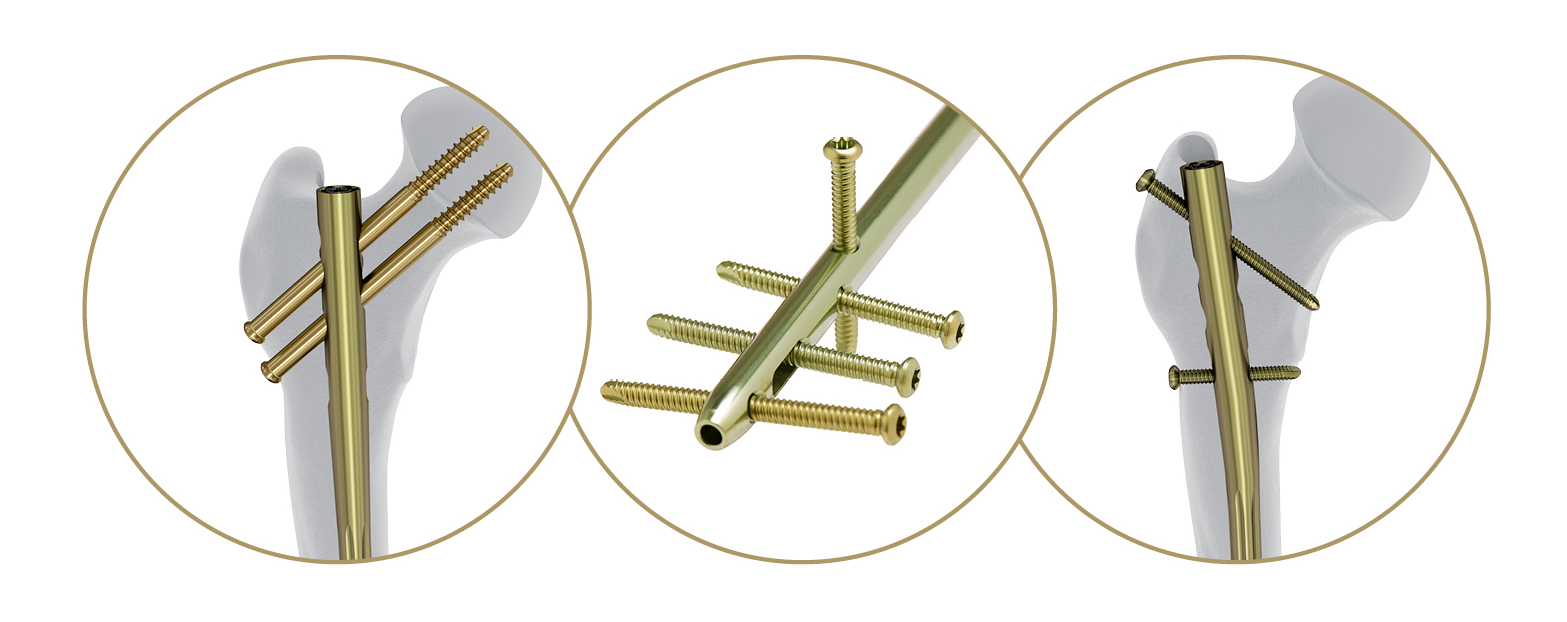
The overall annual number of femoral shaft fractures in the U.S. is estimated at approximately 90,000. The DePuy Synthes Femoral Recon Nail System is a fixation system that utilizes an intramedullary nail to repair femoral shaft fractures. The system provides a surgeon with a state of the art nailing system that is designed to provide stability and allow patients to move soon after surgery while optimizing patient outcomes.
The Femoral Recon Nail System offers surgical entry point and locking options to accommodate varying preferences, and enables surgeons to treat a broad range of complex fractures. The system also features radiolucent insertion handles that aim to reduce surgical complexity, facilitate intraoperative and X-ray visualization, and assist with guide wire placement.
(Image credit: DePuy Synthes)
“I believe the DePuy Synthes Femoral Recon Nail System offers the most streamlined insertion instruments on the market,” says Pete Nowotarski, MD, Professor Orthopaedic Surgery, University of Tennessee College of Medicine, Chattanooga & Orthopaedic Trauma Director, Erlanger Health Systems.” This system, coupled with the most advanced design features in both the piriformis fossa and greater trochanter entry points, offers greater versatility in treating complex femur fractures.”
In addition, the DePuy Synthes system is also designed to address a potential operative complication called distal cortical impingement, which is often the result of the curve of the patient’s femoral anatomy being greater than the curve of the nail. The Femoral Recon Nail System addresses this issue as it is specifically designed to enhance anatomical fit by reducing the rate of nail curvature to more closely match the patient’s anatomy that may also further improve the patient’s experience.
“DePuy Synthes has a long history of innovation in the hip and femur fracture repair market with a comprehensive portfolio of implants that address a variety of patient needs,” says I.V. Hall, Global Platform Leader, DePuy Synthes Trauma. “This launch allows DePuy Synthes to offer the most comprehensive, best-in-class portfolio for hip and femur fractures as we continue to advance the standard of care for patients and improve the surgeon experience.”




