Sam Ruback, Product Manager, Parker Hannifin Corporation
A recent generation of miniaturized pumps and valves is making it easier for design teams to shrink the size of some medical devices. No surprise, making best use of them requires a few engineering tradeoffs to make best use of smaller components.
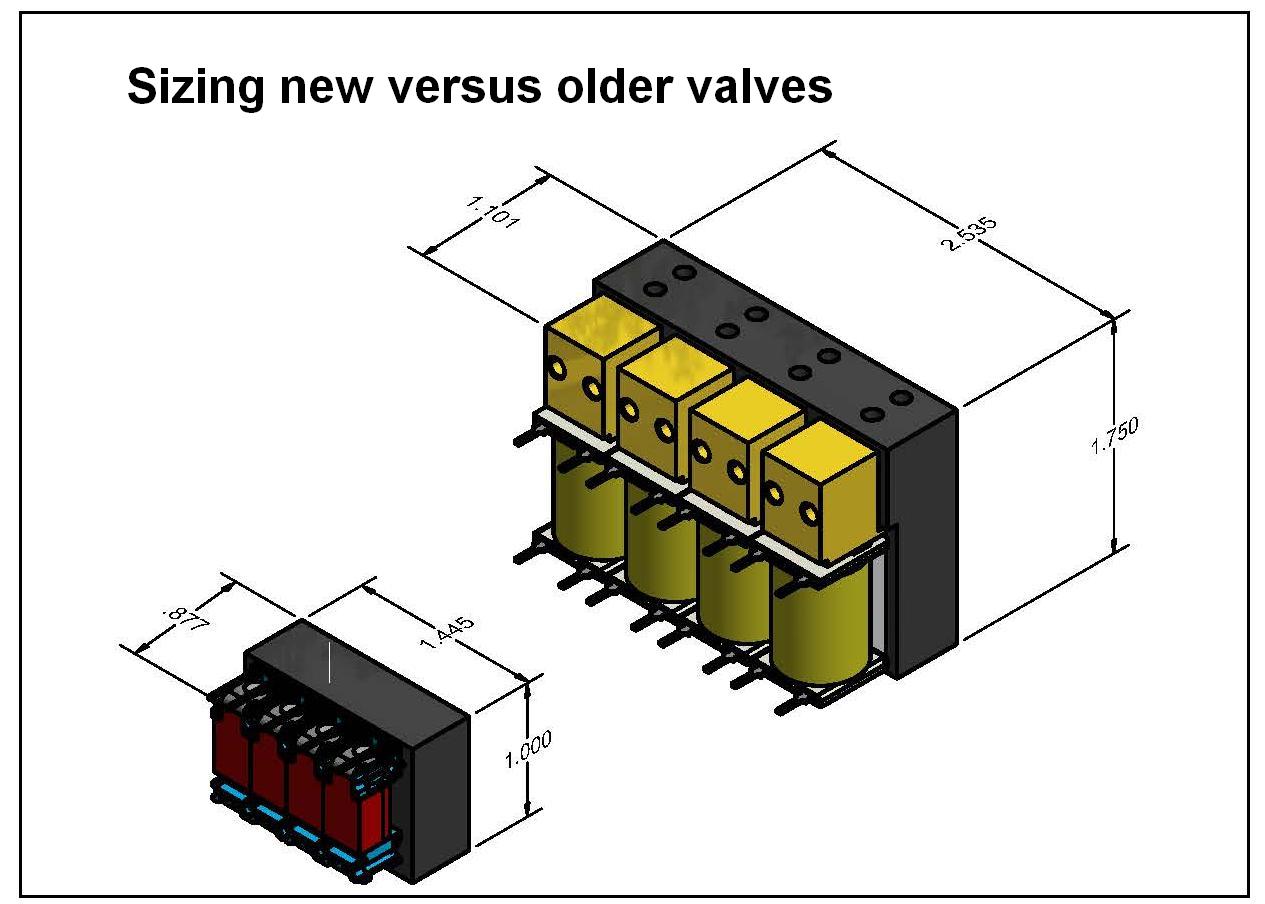
A CAD model shows four Parker S11 model valves (left) on a manifold for comparison to four Parker X model valves on a manifold. Each S11 valve offers an 82% reduction in size and a 93% reduction in weight.
In a nutshell, the engineering challenges to going small include:
- Valves and pumps must consume less power so batteries can be smaller and yet last longer.
- Valves and pumps must better integrate into manifolds to consume less space.
- They cannot sacrifice flow rates, create thermal issues, or increase costs.
Thinking small
Next-generation solenoid valves are considerably smaller than their predecessors. These electro-pneumatic on-off valves are often specified by the system physics, such as flow and pressure.
Additional considerations might be response time, leak, and cost. Comparing an older model format solenoid valve with a newer version from the same manufacturer, may show a size reduction of up to 82% and a weight reduction of up to 93%.

Parker’s VSO LowPro proportional valve sacrifices porting options for small size. It is optimized around a single widely used porting style, in this case, a face-mount style. If a designer wants a customized solution, such as a T-porting style, the company would partner to create a solution.
Proportional valves that offer flow control are not as easy to reduce in size because they need space for the mechanism that allows ramping ramp up and down. Nevertheless, significant miniaturization has been possible, on the order of 63% smaller and 81% lighter.
Pumps offer less opportunity for size reduction. Newer designs are in the range of 10% smaller. Typically, a medical device will have many more valves than pumps, so the overall size and weight reduction can still be significant.
Component manufacturers achieve miniaturization several ways. One of the most important is in the geometry of the pump or valve, such as integrating a flux bracket (magnetic circuit element that lets a magnetic field travel and focus along a specific path to actuate the valve into an open or closed position) into the body and paying attention to the mechanical design of related fluidic components. A few design considerations follow.
Integration flexibility – One engineering tradeoff has to do with plumbing. The smaller geometry of miniaturized components usually provides less space to reconfigure a flow path in a different direction. This limits altering port locations in later iterations of the design.
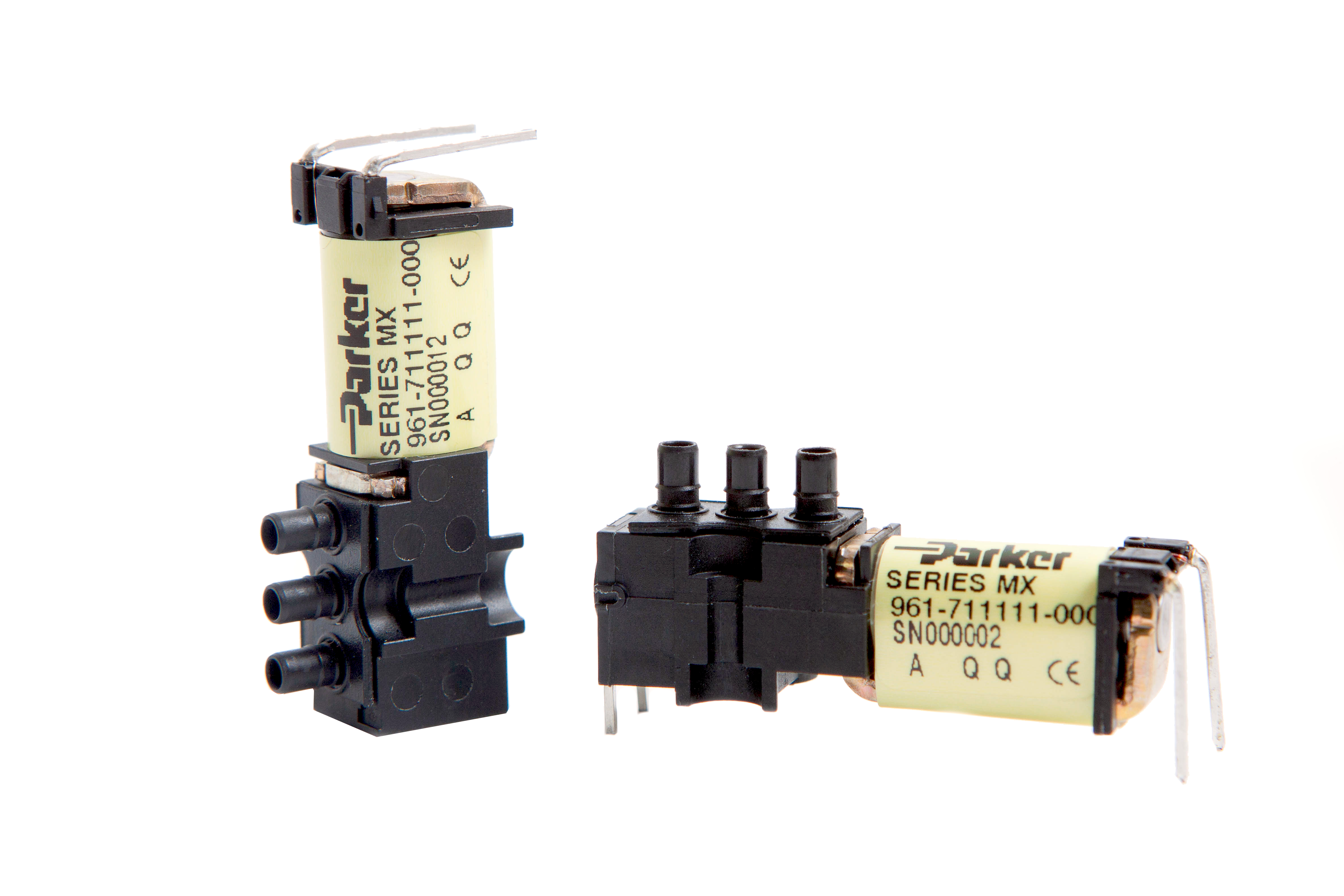
Miniature valves, such as Parker’s MX series, accommodates a variety of porting styles. The miniaturized design sacrifices flow customization. Flow is relatively fixed, as is power.
On the plus side, smaller components may ease integration because the pumps and valves can be easily built into a small PCB or subassembly. For example, if a manifold that mounts three valves must be built into a subsystem, then a smaller size gives more physical space for the engineer to work with.
In some cases, smaller components may not reduce the footprint of a machine, but can free up space for better organization and access to the components. For example, on a large ventilator used for anesthesia in a hospital, a pump and valves may be fit into the back corner of the machine. Smaller parts may make the parts more accessible, which makes the machine easier to build, service, and clean.
Typically an OEM design will ask for components with the same specs as those ordered the last time, just in a smaller package. Filling the order may not be possible. Often, one cannot just take the valve and the pump built into a device 10 years ago and substitute smaller components. Nevertheless, if one or two of the specs are flexible, a significant size reduction is often possible. To make a best selection, it’s important to pick a supplier that can facilitate engineer-to-engineer conversations that dig into the particulars of a project and explore the possible tradeoffs.

Just for comparison, the typical flash drive gives an sense of the size of a Parker VSO LowPro valve.
Keep in mind that miniaturization does not always mean a smaller sub-assembly. It is possible to add more features and maintain the same sub-assembly size. For example, the designer for a compression therapy device wanted to add more zone control selections and a master safety switch. The valve manufacturer worked with the designer and recommended replacing four large valves with six smaller valves on the same size manifold. This provided an extra arm for control and an on-off valve for safety.
Leak rate – A leak rate can be a keystone spec. A low leak rate may be more critical than, say, porting options. In some applications, a dimensionally larger valve will be the only way to meet the leak-rate spec. In fact, sometimes the decision is not to miniaturize. The design may need power efficiency, heat reduction, or quiet operation more than a smaller sub-assembly.
Many OEM designers don’t know what a leak rate spec should be. That can be discussed with the valve manufacturer’s application engineer. In other applications, the OEM designer knows exactly what the leak rate must be. A good example is an operating-room anesthesia machine pumping nitrous oxide. No one wants the gas escaping and affecting the surgeon during an operation. A portable oxygen concentrator has a different story.
Design work – Often, a smaller valve or pump is not a drop-in replacement component. The component manufacturer may have advice and, when the unit volume is high enough, some manufacturers will do design work for the OEM. Some component manufacturers also offer to build the sub-assemblies.
Product life – As you would expect, the product life on a datasheet differs from the product life in an actual application. The designer request may be for 100-million cycle life. But if the medical device only receives occasional use, then 5-million cycles would be sufficient.
Unfortunately, a required life may be an unknown. One may assume product success came from a stringent lifetime spec, when in reality, the product was used only twice a day for 10 years. In addition, engineers love to over specify, especially for medical devices, because the liability of a failure could be catastrophic.
It is also helpful to consider the range of application uses. A diagnostic instrument in a large laboratory needs an extreme cycle life to handle hundreds of tests per day, while a smaller version of the same instrument used in doctors’ offices may handle few tests per day.
It is worth noting that when a valve manufacturer first introduces a new, smaller valve, the company may not have a good handle on expected device lifetime. That’s because the tests that predict lifetime take months to run.
Flow rate – Design teams must often prioritize the maximum operating pressure and flow output. If the ideal spec calls for 20 liters/hr with a 2x safety margin, and the designer wants a smaller subassembly, then meeting the spec may not be possible. Smaller may valves have smaller orifices and operate at higher pressures, so it is hard to balance the tradeoff between flow and pressure while miniaturizing. The question to ask: Is a 2x margin necessary on both pressure and flow?
Power consumption – Power efficiency is the most commonly required feature of portable medical devices. Maximizing battery life might let the device operate longer before it needs a recharge. One suggestion is to evaluate pumps using two different ratios: power versus size and power versus flow. Smaller pumps may, in some cases, use the same amount of power but move less fluid.
Heat – Its dissipation is a common tradeoff of miniaturization, simply because smaller components have less mass for heat dissipation. That said, the situation depends on the specific component. Many smaller components operate at lower temperatures.
Every application is different
If there is a rule of thumb, it is that there are no rules of thumb. Some tiny pumps and valves run cooler and cost less than their larger counterparts, and some have all the specs that a design needs. What’s more, engineering tradeoffs for valves differ from those for pumps. The best advice is to examine all the specs and know in advance where one can be flexible. Understand the difference between needs and wants.
OEMs looking for answers may depend heavily on the pump and valve manufacturer. Often the best partner is one who has a deep product line and willingness to help specify, design, build, assemble, test, calibrate, and ship. Such value-added services let the OEM concentrate on their core business. Another thing: Look for a partner ISO 13485 certified because that shows familiarity with the requirements of devices used in life science.