Michele Coleman lay close to death last fall with end-stage lung disease when she got a second chance at life.
Thanks to an innovative approach aimed at broadening the pool of lung donors, Coleman, 63, received a new set of lungs that previously had been deemed unacceptable for transplant.
As part of a clinical trial at Washington University School of Medicine in St. Louis, lung transplant surgeons are evaluating whether a sophisticated device can recondition subpar donor lungs to make the organs suitable for transplant. The lungs Coleman received – during surgery at Barnes-Jewish Hospital – were connected to a machine designed to evaluate and enhance lung function by ventilating and perfusing the organ for up to six hours after the lungs’ retrieval from a donor.
The device has the potential to expand the number of donor lungs that can be transplanted, potentially helping the 1,480 patients currently waiting for lung transplants.
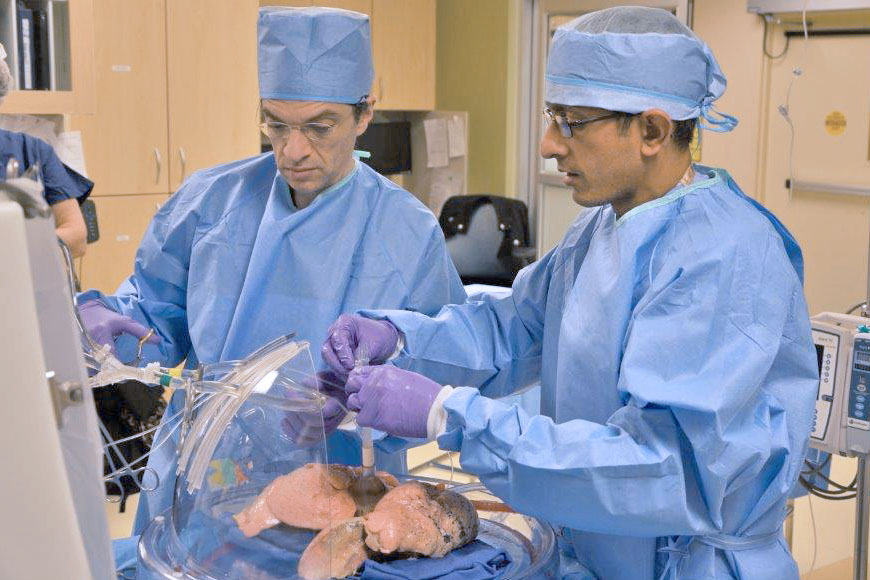
Surgeons at Washington University School of Medicine in St. Louis recondition lungs in a device — housed at Mid-America Transplant — that has the potential to expand the lung transplant donor pool. Conducting the procedure are lung transplant surgeons (left) Daniel Kreisel, MD, PhD, and Varun Puri, MD. (Credit: Mid-America Transplant)
Washington University is one of 16 U.S. medical institutions participating in the trial, which will evaluate the effectiveness of the lung device. About 250 patients nationwide will be enrolled. The technology already is being used in Europe and Canada.
“Currently, many more patients need lung transplants than there are available donors,” said Daniel Kreisel, MD, PhD, professor of surgery and of pathology and immunology at the School of Medicine, and the study’s principal investigator. “We are hopeful that the device can significantly expand the lung donor pool and save more lives.”
In 2015, about 360 people on the lung-transplant waiting list died or became too ill to receive a donor organ, according to the United Network for Organ Sharing (UNOS), which manages the U.S. organ transplant system.
Two years ago, the U.S. Food and Drug Administration unanimously approved the lung device for use in cases when patients with end-stage lung disease have no other options. “The FDA recognizes that the machine is relatively safe,” said Kreisel, surgical director of the medical school’s lung transplant program. “But further studies are needed before the device can be used widely to aid lung transplant patients.”
In St. Louis, the device is housed at Mid-America Transplant, whose staff is trained to closely monitor the lungs as they are reconditioned.
Spongy and elastic, lungs are one of the most difficult organs to transplant, with only about 20 percent of the organs meeting clinical transplant standards, said Kreisel. The organ’s fragility also means lungs are susceptible to injuries and infections and, therefore, frequently are deemed unsuitable for transplant.
The device maintains lungs in a “body-like” environment after a donor’s death, by circulating a nutrient solution through the lungs and ventilating them at body temperature. The sterile, controlled setting, close monitoring and lengthened recovery time – between four and six hours – allow surgeons to have an extended and thorough assessment of the lungs and provides time for the organs to recover from the inflammatory shock following brain death.
About half of the lungs assessed after being reconditioned in the device meet eligibility standards for transplant, Kreisel said. These include previously unsuitable lungs from older donors and lungs donated after cardiac arrest, which stops blood flow to the lungs sooner than brain death.
Coleman, who received her double-lung transplant in November, was the School of Medicine’s first patient to receive lungs that had been reconditioned in the device. Before her transplant, chronic obstructive pulmonary disease left her exhausted, mostly bedridden and reliant on an oxygen tank.
“Lung transplants are a high-risk procedure, as are all organ transplants,” said Varun Puri, MD, an associate professor of surgery at the School of Medicine who, along with Kreisel, operated on Coleman.
Coleman knew she could die during the surgery Nov. 17. “But I had nothing to lose,” she said. “I was ecstatic for the chance. Without the transplant, I am sure I would have died by Christmas.”
More than four months later, Coleman is breathing independently as well as showering and dressing herself without having to stop for frequent rests. She can walk again, and does so for at least 30 minutes a day to build strength.
“Michele has been recovering very well,” said her physician, Ramsey Hachem, MD, professor of medicine and the medical director of lung transplantation at Washington University.
“Nov. 17 marked my new beginning,” Coleman said. “I was given a whole new life.”




