Cyanoacrylate topical skin adhesives
Cyanoacrylate topical skin adhesives (TSA) have been cleared for use in the U.S. for over a decade and are indicated for topical applications only, to hold closed easily approximated skin edges of wounds from surgical incisions, including punctures from minimally invasive surgery and simple, thoroughly cleansed, trauma induced lacerations.1 TSAs may be used in conjunction with, but not in place of, deep dermal stitches. Used throughout the world for their effective wound closure properties, TSAs continue to gain acceptance as effective alternatives to conventional suture and staple closures in a wide variety of medical applications.2 The products have a variety of benefits over conventional closure techniques—both for the clinician and the patient.
TSAs provide atraumatic wound closure, faster closure times and similar to better cosmetic outcomes3 compared to traditional wound closure techniques such as sutures and staples. In addition to their proven use as wound closure devices, TSAs are also increasingly used as surgical site microbial barriers to protect the closed wound from microbial contamination after primary closure. They have been proven to provide effective contaminant barriers as long as the adhesive film remains intact. There is clinical evidence that demonstrates reduced wound infection from their use.4-8
Surgical Site Infections
Microbial contamination of the surgical site is a necessary precursor of surgical site infection (SSI). A recent prevalence study found that SSIs were the most common healthcare-associated infection, accounting for 31 percent of all healthcare-associated infection among hospitalized patients.9 It is also estimated that over 2 percent of all patients admitted for a surgical procedure will develop a surgical site infection.10 The Center for Disease Control (CDC) healthcare-associated infection prevalence survey found that there were an estimated 157,500 surgical site infections associated with inpatient surgeries in 2011.11 Data from the CDC’s National Healthcare Safety Network (NHSN) included 16,147 SSIs following 849,659 operative procedures in all groups reported, for an overall SSI rate of 1.9 percent between 2006–2008.12
As a result of these infections, patient hospitalization time is extended and the overall cost of care increases by up to 2.9 times.13 While advances have been made in infection control practices, SSIs remain a substantial cause of morbidity, prolonged hospitalization, and death. SSI is associated with a mortality rate of 3 percent14, and 75 percent of SSI-associated deaths are directly attributable to SSI.15
CDC Guidelines for the prevention of surgical site infection recommend that a wound is kept covered with a sterile product for 24–48 hours post primary closure to reduce the risk of infection.16 After this time, the CDC is unclear whether an incision needs to be covered as the body’s natural healing mechanisms commence and begin to provide its own protection from microorganisms.
Devices or practices that may provide a barrier to microorganism entry into the surgical site can be utilized to help improve patient recovery outcomes4,7,17,20,21 and to help reduce the financial burden placed upon care facilities.9,11,13 Preventive strategies, such as providing an effective barrier to infection, may help alleviate this considerable strain to healthcare resources.
TSAs, therefore, may help provide not only primary closure but also an effective microbial barrier throughout this critical time period. Reduced infection rates have been reported when cyanoacrylate topical skin adhesives are used on top of wounds primarily closed with sutures. For instance, Souza et al. reported a reduction from 4.9 percent down to 2.1 percent in infection rates for cardiovascular surgery patients. When comparing the median postoperative hospital stay of patients, those patients whose wounds were closed via conventional sutures plus TSA cited a lower length of hospital stay versus patients whose wounds were closed using conventional sutures only.17
Proven effectiveness of TSAs as microbial barrier
To prove microbial barrier effectiveness of TSAs, manufacturers utilize industry standard testing methodology and submit to the FDA during the product review process. The testing includes subjecting the adhesive layer to contact with various organisms known to cause surgical site infections.
Test results for Cardinal Health™ LiquiBand OCTYL Topical Skin Adhesive
To demonstrate the microbial barrier properties of Cardinal Health™ LiquiBand® OCTYL Topical Skin Adhesive, an in vitro evaluation was conducted using methodology established as industry standard. The product was applied to a pre-defined area on agar test plates (n=100), followed by the application of a highly concentrated titer of selected microorganism. A change in color of the agar would indicate a breach of the adhesive layer. Plates were examined for any change at day three and day seven. The chart below displays the percent maintenance of the microbial barrier per test organism at day three and day seven after inoculation. Over all species, the product maintained a barrier in 899 out of 900 total plates tested at day three, and 881 out of 900 plates tested at day seven.18 These results indicate that Cardinal Health™ LiquiBand® OCTYL Topical Skin Adhesive provides an effective barrier to microbial penetration.
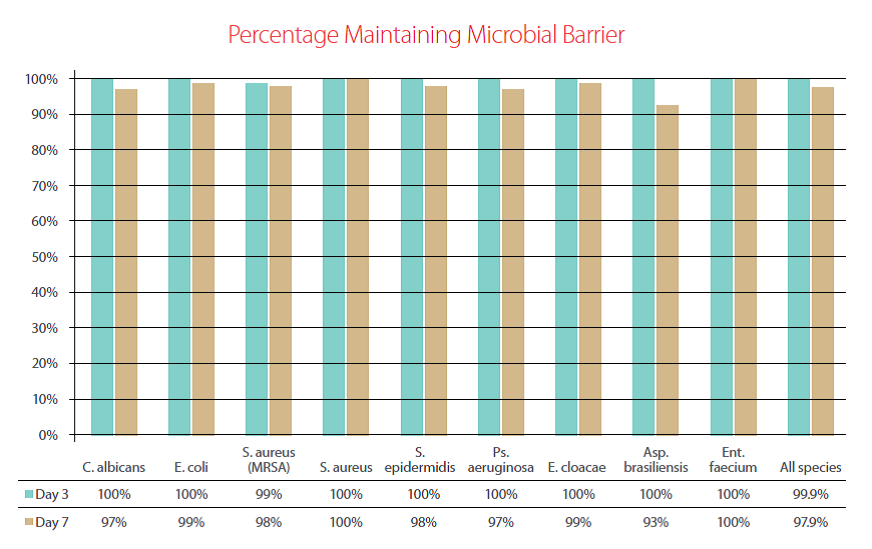
Chart 1: Microbial barrier testing performed per industry standard testing to demonstrate the microbial barrier properties of Cardinal Health™ LiquiBand® OCTYL Topical Skin Adhesive at day 3 and day 7 post-inoculation with various species of gram positive, gram negative, fungal and mold species.
Discussion
There are many studies evaluating the microbial barrier properties of TSAs, two of which cite Bhende et al.19 and Brown20 who examined the microbial barrier properties of two other topical skin adhesive products — Dermabond® 2-octyl cyanoactylate (Ethicon Inc.), and Indermil™ n-butyl cyanoacrylate (Covidien) respectively. All three products have demonstrated microbial barrier properties. In comparison to Bhende’s methodology, Cardinal Health™ LiquiBand® OCTYL Topical Skin Adhesive was challenged for a longer duration of time and with higher microbial challenge levels (7 days, >105 cfu/mL).
A recent animal study by Karatepe et al.8 investigated the usefulness of cyanoacrylate in preventing early wound contamination; this study demonstrated that maintaining skin integrity and providing a barrier to microbe entry for the first twenty-four hours is critical to prevent infection after skin closure. It also demonstrated that, after this period, the natural tissue healing process has commenced, and therefore the skin starts to provide its own microbial barrier function. In the control group, (sutures) all the hernia grafts were infected after being subjected to a microbial challenge within 24 hours of closure. In the study arm, (cyanoacrylate) no graft infections were noted after microbial challenge within 24 hours of closure. After 24 hours, the microbial challenge was repeated; no difference was found between the number of infections closed with sutures, versus those closed with cyanoacrylate. These findings are in line with the CDC guidelines on preventing surgical site infections which recommend that a surgical wound be protected with a sterile dressing for 24 to 48 hours postoperatively following primary closure.9 Previous studies have demonstrated that within 48–72 hours of wound closure, the natural wound healing cycle results in an effective microbial barrier.21,22 Hence, it is within the first 24–48 hours that the microbial barrier properties of TSA are most critical.
A wound closure device that provides an effective barrier for up to 72 hours should provide sufficient time to allow for the natural wound healing process. The in-vitro microbial barrier findings highlight how TSAs may be used to help protect against microbial contamination during this critical time.
Citations
1. 21 CFR 878.4010
2. Liebelt, E. Current concepts in laceration repair. Curr Opin Pediatr. 1997: 5: 459-464.
3. Quinn JV, Drzewiecki, Li MM, Stiell IG, Sutcliffe T, Elmslie TJ, Wood WE: A randomized, controlled trial comparing a tissue adhesive with suturing in the repair of pediatric facial lacerations. Ann Emerg Med 1993, 22:1130-1135.
4. Quinn, J. Maw, J. Ramotar, K. Wenckebach, G. Wells, G. Octylcyanoacrylate tissue adhesive versus suture wound repair in a contaminated wound model. Surgery. 1997 Jul: 122 (1): 69-72.
5. Gristina, A.G. Price, J.L. Hobgood, C.D. Webb, L.X. Costerton, J.W. Bacterial colonization of percutaneous sutures. Surgery. 1985 Jul; 98 (1): 12-9.
6. Sönmez, K. Bahar, B. Karabulut, R. Gülbahar, O. Poyraz, A. Türkyilmaz, Z. Sancak, B. Basaklar, A.C. Effects of different suture materials on wound healing and infection in subcutaneous closure techniques. BENT. 2009; 5 (3):
149-52.
7. Aksoy, M. Turnadere, E. Ayalp, K. Kayabali, M. Ertugrul, B. Bilgic, L. Cyanoacrylate for wound closure in prosthetic vascular graft surgery to prevent infections through contamination. Surg Today. 2006; 36 (1): 52-6.
8. Karatepe, O. Ozturk, A. Koculu, S. Cagatay, A. Kamali, G. Aksoy, M. To what extent is cyanoacrylate useful to prevent early wound infections in hernia surgery? Hernia. 2008 Dec; 12 (6): 603-7. Epub 2008 Jun 5.
9. Magill, S.S., et al. “Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida”. Infection Control Hospital Epidemiology, 33(3): (2012): 283-91.
10. Klevens, M et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Reports. March–April 2007. Volume 122: 160-166
11. Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med 2014; 370:1198-208.
12. Mu, Y., et al. “Improving risk-adjusted measures of surgical site infection for the national healthcare safety network”. Infection Control Hospital Epidemiology, 32(10): (2011): 970-86.
13. Perencevich EN, Sands KE, Cosgrove SE, Guadagnoli E, Meara E, Platt R. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis [serial online] 2003 Feb [date cited]. Available at: http://www.cdc.gov/ncidod/EID/vol9no2/02-0232.htm. Accessed 11 December, 2009.
14. Awad, S.S., “Adherence to surgical care improvement project measures and post-operative surgical site infections”. Surgical Infection (Larchmt), 13(4): (2012):234-7.
15. Anderson DJ, Kaye KS, Classon D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol 2008;29:S51–S61.
16. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, the Hospital Infection Control Practices Advisory Committee (CDC). Guideline for the prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol . 1999;20:247-280
17. Souza EC, Fitaroni RB, Januzelli DM, Macruz HM, Camacho JC, Souza MR. Use of 2-octyl cyanoacrylate for skin closure of sternal incisions in cardiac surgery: observations of microbial barrier effects. Curr Med Res Opin. 2008 Jan; 24(1):151-5.
18. Data on file at Advanced Medical Solutions (Plymouth) Ltd. Test report 000-928 Report on Microbial Barrier Properties
19. Bhende S, Rothenburger S, Spangler DJ, Dito M. In Vitro assessment of microbial barrier properties of Dermabond® topical skin adhesive. Surgical Infections. 2002. 3(3):251-7
20. Brown L. Effectiveness of Indermil® tissue adhesive as a Microbial Barrier. Available at: http://www.covidien.com/ imageServer.aspx?contentID=821&contenttype=application/pdf. Accessed 11 December, 2009.
21. Rosevear C, Dott J, Lazarus R. Reducing Risk Of Post-Operative Complications After Joint Replacement Surgery, Nurse Unit Manager Periopertive Services, Geelong Private Hospital, Geelong, Victoria 3220, Australia.
22. Nursing Times, Surgical wound care: current views on minimizing dressing-related pain, (September, 2004).




