Many of today’s medical devices rely on electric actuators for motion control in critical, life-saving applications. They offer a number of advantages that suit this industry, including their performance and increased efficiency. Clean operation—operating without the need for fluids or ancillary equipment—is one of the major advantages of electric actuators.
Electric actuators use roller screw technology, which enables high load capacity (more than 100,000 lb). Load capacity and rigidity of planetary roller screws suit applications requiring continuous force. Yet, these devices operate quietly. The planetary design of roller screws allows operations at higher rotational speeds than other screw technologies, useful in high-speed applications.
Another benefit of electric actuators is the ability to integrate power, control, and actuation mechanisms into one device. Electric actuators combine force, velocity, and positioning in a single, compact motion control device.
Actuator size constraints can be problematic, as medical devices require building to precise specifications. Innovations in motor technology offer smaller package sizes, but with the same power output as previous motor designs. Integrating the motor and linear actuators into one package reduces the overall size of the actuator, enabling it to install easily into equipment or other applications using minimal space.
In a compact design, electric actuators also offer the option of integrating the electric controls and power circuitry. Such a configuration eliminates external components and expensive cables. These actuators operate without a stationary electrical cabinet. Thus, allowing the actuator, drive, and control to operate as a stand-alone piece of equipment.
By combining all crucial motion control components into the actuator, you can design mobile medical devices that offer the same capabilities common in stationary applications.
Medical devices are only as reliable as their components. Electric actuators deliver dependable motion control in diverse situations ranging from simple solutions to critical, lifesaving applications that demand precise positioning, smooth motion, and tightly controlled velocity.
For example, directed radiation therapy, used commonly as a part of cancer treatment programs, applies radiation to patients in progressive increments, slice-by-slice, delivered to different sections of a tumor. During this treatment, the position of the patient must be beneath a precise, fixed source of radiation. To accommodate this, electric actuators manipulate the position of the patient’s bed, a critical aspect of the application.
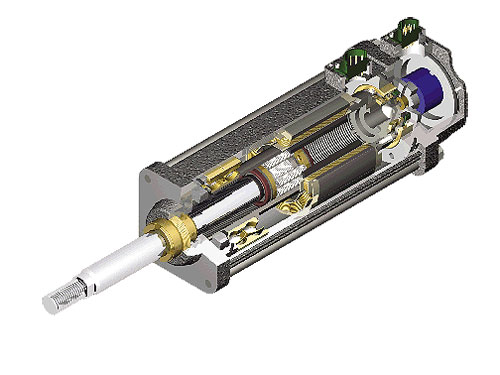
Electric actuators use roller screw technology, which enables high load capacity (more than 100,000 lb). Load capacity and rigidity of planetary roller screws suit applications requiring continuous force. Yet, these devices operate quietly, which helps in medical applications.
Not only does the actuator provide the positioning necessary in these applications, it also delivers the smooth motion and velocity control necessary for proper radiation application. Featuring high force density, certain electric actuators are easily concealed within these types of devices, minimizing space requirements and enabling a piece of equipment, like a patient table, to feature a logical design without excessive size to contain oversized actuators.
In addition to performance and size, electric actuators also minimize the noise associated with operation. Studies show that when patients can hear noise from medical equipment during a procedure, there is a noticeable increase in their anxiety. Due to the proximity of the actuator to the patient, any audible noises produced during operation are easy to hear, creating unnecessary nervousness during medical procedures. Therefore, implementing electric actuators with roller screw technology, which offers significantly less noise than alternative actuators, assures quiet operation, increases patient relaxation and reduces apprehension during this necessary and intricate treatment.
Exlar
www.Exlar.com





