Using a properly formatted “roadmap” can help a medical device development team realize that the regulations are structured to yield innovation rather than stunting it.
Christopher Montalbano, MIDI Medical Product Development
(Image courtesy of MIDI Medical Product Development)
The complicated process of satisfying FDA requirements gives many medical device developers pause.
This stems from the FDA not only prescribing the detailed method for putting a medical device through its development process, but also requiring the company(ies) associated with the development to embed this process into their essential DNA — documenting it within the organization’s quality management system (QMS).
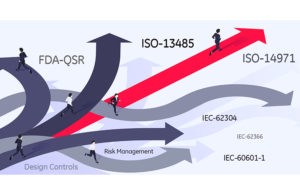
The QMS should describe and provide a visual roadmap so an organization can consistently deploy innovation in their engineering development work.
The best way to explain the roadmap and how it leads to innovation is to open up the map, view the entire scope and discuss the 3 key stops along this innovation journey. The maturity of a team’s medical device development program will determine if a company deploys extensive activities at each of these stops, or in some instances, pauses briefly to check there is enough intelligence and collateral knowledge in place before moving on to the next stop.
Stop-1: Market exploration & discovering opportunities
The FDA guidance recognizes this as a quintessential activity for any business, yet they make it known that their regulatory controls such as Design Controls & Risk Management do not have to be performed at this point.
In Stop 1, you set the stage for establishing a value proposition between the internal stakeholders (the corporation) and the external stakeholders such as doctors, patients, purchasing decision-makers and payors.
It’s important to identify and explore external needs. This is often what unlocks innovation and makes your company stand out from the competition.
Stop-2: The technology R&D process
The emphasis at this stop is on iteration, testing and incorporating feedback between iterations or “agile sprints.” Each sprint is an opportunity to advance the device both iteratively and incrementally. The idea behind an iterative agile sprint process is to innovate, design and engineer proof of concept and minimum viable product (MVP) of the device and/or technology application. The express purpose is to capture feedback both internally and externally from multiple perspectives.
MVP test results should indicate if the device meets the overall criteria set forth in the previous stop on the roadmap. This approach fosters a quality feedback loop for system function, reliability and throughput.
Stop 3: Commercialization and implementation
Now is the time the development team needs to pay attention to ISO-13485.
I encourage medtech companies to view the standard not as a constraint, but rather as a guide to being systematic and well-documented, allowing you to standardize the process and free your teams to focus on implementing and commercializing their innovations. Standardizing the process requires a robust QMS to define in detail the work that needs to be done.
The team now starts the process of capturing and mining user requirements to develop the more refined aspects of device innovation. It breaks the user requirements down into device requirements or specific feature definitions
User requirements must be decomposed into device requirements, or specific feature definitions. Mapping out goals and using customer-focused development can yield innovative device features, unlocking new intellectual property that can enhance device value.
Christiopher Montalbano is co-founder and CEO of MIDI Medical Product Development. He is a member of the Stony Brook University Center for Biotechnology advisory board and has served as a guest judge for BiomedX at Columbia University and as a Texas Medical Center Innovation External Institute Advisors.
The opinions expressed in this blog post are the author’s only and do not necessarily reflect those of Medical Design and Outsourcing or its employees.




