A look at IEC 60601-1-2 4th edition requirements
By Ron Stull, Power product marketing engineer
While the original IEC 60601-1 standard has existed now for 40 years, technology has evolved and the environments where medical equipment is used has grown more complex. This has led to the consideration of risks due to electromagnetic interference and a collateral standard on electromagnetic compatibility.
In the following post we will take a look at IEC 60601-1-2 4th edition EMC collateral standard, how it relates to medical power supplies, and its effective date in each region.
IEC 60601-1-2 4th edition requirements
The underlying premise of IEC 60601-1 is understanding and managing risk, which the 3rd edition developed by defining electrical performance requirements for safe operation in terms of the means of protection for both patients and operators. Principally this determined isolation, creepage and insulation specifications for different classes of use. It should also be noted that edition 3.1 of the standard clarified some earlier definitions in the light of newer technologies.
The 4th edition EMC collateral standard maintains this focus on risk analysis, but moves away from equipment categories, such as “life-supporting,” and instead considers “intended use environments.” Specifically, it defines professional healthcare, home healthcare, and “special” environments. Professional covers the traditional use of medical equipment in hospitals and similar places where medical staff are present, but which these days are also subject to increased EMC challenges. Home use deals both with the requirements of non-specialist users as well as with situations where electrical supplies may be less stable. Special is a contingency for environments that may present high levels of electromagnetic disturbance, including industrial locations or in a radio-therapy treatment room.
Providing immunity to the sources of interference these environments may produce is the key to the more rigorous test specifications defined in the 4th edition, as the table outlines below.
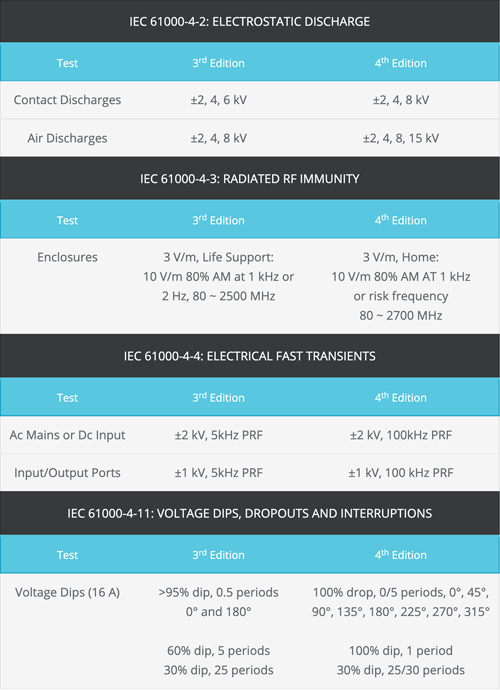
Key changes to immunity test levels from 3rd edition to 4th edition of IEC 60601-1-2
IEC 60601-1-2 4th edition and power supplies
Outside of battery-powered devices, it is not really possible to exclude power supplies from the IEC 60601-1 regulations that apply to medical equipment. This is evident from the previous 3rd edition classifications for patient and operator protection where a distinction is made between equipment in the same room as a patient, versus equipment that makes physical contact with the patient, especially in the case of contact with the patient’s heart, as with a defibrillator.
In the latter case, only IEC 60601-1 qualified supplies are acceptable and even then, an additional isolation barrier is required between the supply and the point of contact with the patient. Even excluding cardiac equipment, the isolation requirements for other uses are likely to impinge on the specification and performance of a power supply.
Likewise, the immunity requirements defined by the 4th edition impact power supply design, so equipment designers are best served to select medical grade power supplies that comply both with IEC 60601-1 edition 3.1 and the 4th edition EMC standards.
Compliance with IEC 60601-1-2 4th edition
The global timeline for compliance with the various editions of IEC 60601-1, including the 4th edition EMC standards is fully detailed here. However, in broad terms, edition 3.1 is currently in force in the US, Canada, Europe, Japan, Korea, and Brazil. Other countries in Asia and elsewhere may be lagging as far as compliance for domestic use is concerned, but if manufacturers are producing equipment for export they will have to ensure compliance in the end-user country.
This same argument applies to the need for compliance with the 4th edition EMC standard which went into effect on a harmonized timeline in the US, Canada and Europe, from January 2019. For an equipment maker who is sourcing its power supplies from another manufacturer, the best solution to keeping on top of complex, evolving standards is to buy ready-qualified units. CUI offers both embedded and external power supplies from 6 watts to 550 watts that are fully compliant with the 4th edition EMC requirements of IEC 60601-1. By selecting one of these pre-certified models you can lessen the burden of ensuring compliance when it comes to medical device design.
Sponsored content by CUI