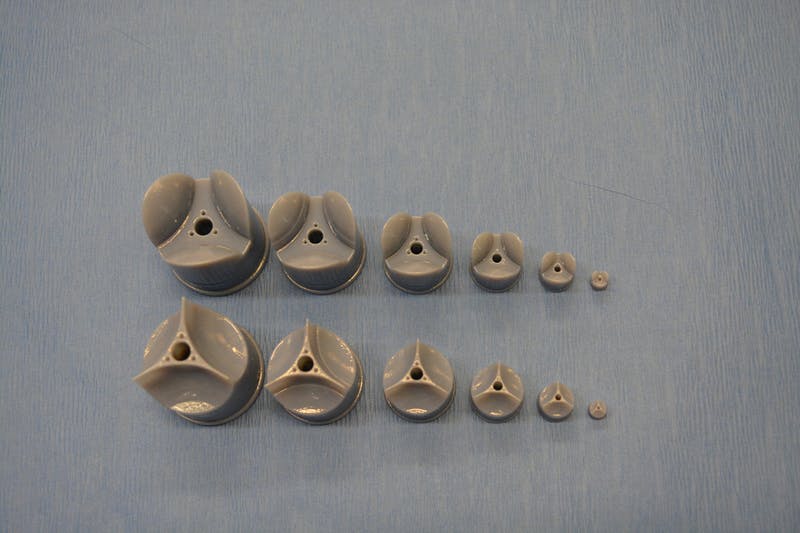
In rotary jet spinning technology, a rotating nozzle extrudes a solution of extracellular matrix (ECM) into nanofibers that wrap themselves around heart valve-shaped mandrels. By using a series of mandrels with different sizes, the manufacturing process becomes fully scalable and is able to provide JetValves for all age groups and heart sizes. (Credit: Wyss Institute at Harvard University)
The human heart beats approximately 35 million times every year, effectively pumping blood into the circulation via four different heart valves. Unfortunately, in over four million people each year, these delicate tissues malfunction due to birth defects, age-related deteriorations, and infections, causing cardiac valve disease.
Today, clinicians use either artificial prostheses or fixed animal and cadaver-sourced tissues to replace defective valves. While these prostheses can restore the function of the heart for a while, they are associated with adverse comorbidity and wear down and need to be replaced during invasive and expensive surgeries. Moreover, in children, implanted heart valve prostheses need to be replaced even more often as they cannot grow with the child.
A team lead by Kevin Kit Parker, Ph.D. at Harvard University’s Wyss Institute for Biologically Inspired Engineering recently developed a nanofiber fabrication technique to rapidly manufacture heart valves with regenerative and growth potential. In a paper published in Biomaterials, Andrew Capulli, Ph.D. and colleagues fabricated a valve-shaped nanofiber network that mimics the mechanical and chemical properties of the native valve extracellular matrix (ECM). To achieve this, the team used the Parker lab’s proprietary rotary jet spinning technology – in which a rotating nozzle extrudes an ECM solution into nanofibers that wrap themselves around heart-valve-shaped mandrels. “Our setup is like a very fast cotton candy machine that can spin a range of synthetic and natural occurring materials. In this study, we used a combination of synthetic polymers and ECM proteins to fabricate biocompatible JetValves that are hemodynamically competent upon implantation and support cell migration and re-population in vitro. Importantly, we can make human-sized JetValves in minutes – much faster than possible for other regenerative prostheses,” says Parker.
To further develop and test the clinical potential of JetValves, Parker’s team collaborated with the translational team of Simon P. Hoerstrup, M.D., Ph.D., at the University of Zurich in Switzerland, which is a partner institution with the Wyss Institute. As a leader in regenerative heart prostheses, Hoerstrup and his team in Zurich have previously developed regenerative, tissue-engineered heart valves to replace mechanical and fixed-tissue heart valves. In Hoerstrup’s approach, human cells directly deposit a regenerative layer of complex ECM on biodegradable scaffolds shaped as heart valves and vessels. The living cells are then eliminated from the scaffolds resulting in an “off-the-shelf” human matrix-based prostheses ready for implantation.
In the paper, the cross-disciplinary team successfully implanted JetValves in sheep using a minimally invasive technique and demonstrated that the valves functioned properly in the circulation and regenerated new tissue. “In our previous studies, the cell-derived ECM-coated scaffolds could recruit cells from the receiving animal’s heart and support cell proliferation, matrix remodeling, tissue regeneration, and even animal growth. While these valves are safe and effective, their manufacturing remains complex and expensive as human cells must be cultured for a long time under heavily regulated conditions. The JetValve’s much faster manufacturing process can be a game-changer in this respect. If we can replicate these results in humans, this technology could have invaluable benefits in minimizing the number of pediatric re-operations,” says Hoerstrup.
In support of these translational efforts, the Wyss Institute for Biologically Inspired Engineering and the University of Zurich announced today a cross-institutional team effort to generate a functional heart valve replacement with the capacity for repair, regeneration, and growth. The team is also working towards a GMP-grade version of their customizable, scalable, and cost-effective manufacturing process that would enable deployment to a large patient population. In addition, the new heart valve would be compatible with minimally invasive procedures to serve both pediatric and adult patients.
The project will be led jointly by Parker and Hoerstrup. Parker is a Core Faculty member of the Wyss Institute and the Tarr Family Professor of Bioengineering and Applied Physics at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS). Hoerstrup is Chair and Director of the University of Zurich’s Institute for Regenerative Medicine (IREM), Co-Director of the recently founded Wyss Translational Center Zurich and a Wyss Institute Associate Faculty member.
Since JetValves can be manufactured in all desired shapes and sizes, and take seconds to minutes to produce, the team’s goal is to provide customized, ready-to-use, regenerative heart valves much faster and at much lower cost than currently possible.
“Achieving the goal of minimally invasive, low-cost regenerating heart valves could have tremendous impact on patients’ lives across age-, social- and geographical boundaries. Once again, our collaborative team structure that combines unique and leading expertise in bioengineering, regenerative medicine, surgical innovation and business development across the Wyss Institute and our partner institutions, makes it possible for us to advance technology development in ways not possible in a conventional academic laboratory,” says Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children’s Hospital, as well as Professor of Bioengineering at SEAS.




