IluminOss Medical has completed enrollment for the first U.S. clinical trial towards U.S. Food and Drug Administration (FDA) approval of its IlluminOss System. The system supports the treatment of fractures using patient-specific intramedullary implants.
The procedure incorporates the use of a thin-walled PET balloon infused with a liquid monomer and inserted into the intramedullary canal of the bone. This conforms to the shape of the patient’s specific bone. The device forms a hardened implant once the surgeon activates the visible light delivered within the PET balloon. Once cured, the implant provides longitudinal strength and rotational stability over the length of the implant.
The IlluminOss implant, which has been approved for clinical use internationally since 2010, was manufactured as an alternative to the nails and plates that are currently used.
“I’m very excited by where this can go in the future, pending FDA approval, as I think this technology could allow us to treat conditions we formerly could not, and can offer ways to treat cancer in the skeleton that were previously impossible,” said Dr. Richard McGough, Chief of Musculoskeletal Oncology at the University of Pittsburgh Medical Center.
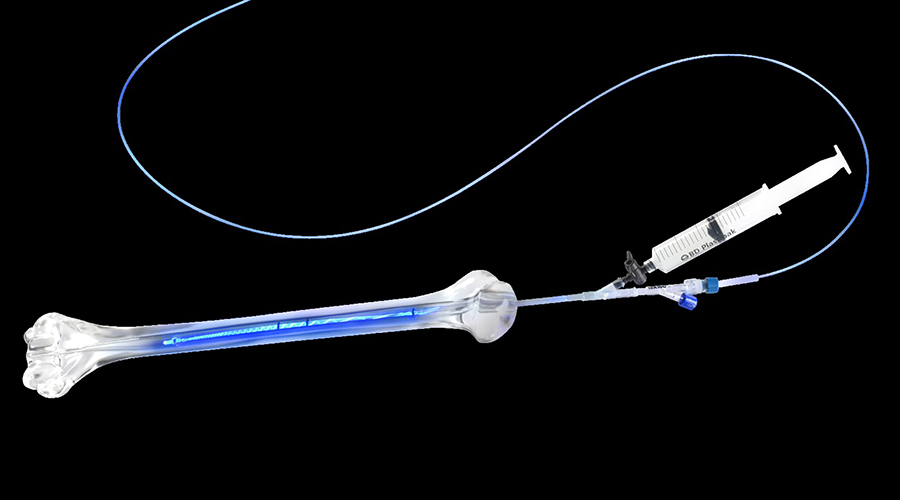
The IlluminOss System (Credit: IlluminOss Medical)
Thirteen surgical sites across the country—including University of Pittsburgh Medical Center, Duke University Medical Center, and Stanford University Medical Center—were involved in the trial, which included 80 patients, all of which had impending or pathologic fractures in their humerus due to metastatic carcinoma.
“We are pleased to have completed enrollment in our U.S. trial and exceedingly satisfied with the feedback we have received so far from participating surgical sites,” said Manny Avila, president and CEO of IlluminOss. “Consistent with what we have observed in international use cases, the IlluminOss System has demonstrated benefits to both orthopedic surgeons and their patients.”
The clinical outcomes data collected will allow IlluminOss to submit a DeNovo marketing application to the FDA and seek marketing clearance for its advanced fracture repair solution in the U.S. in 2017.




