Ethicon has announced that they’re ready to bring the Harmonic HD 1000i Shears to the market, three months after the device received FDA clearance. The official launch took place at American College of Surgeons’ Annual Clinical Congress, in Washington, D.C.
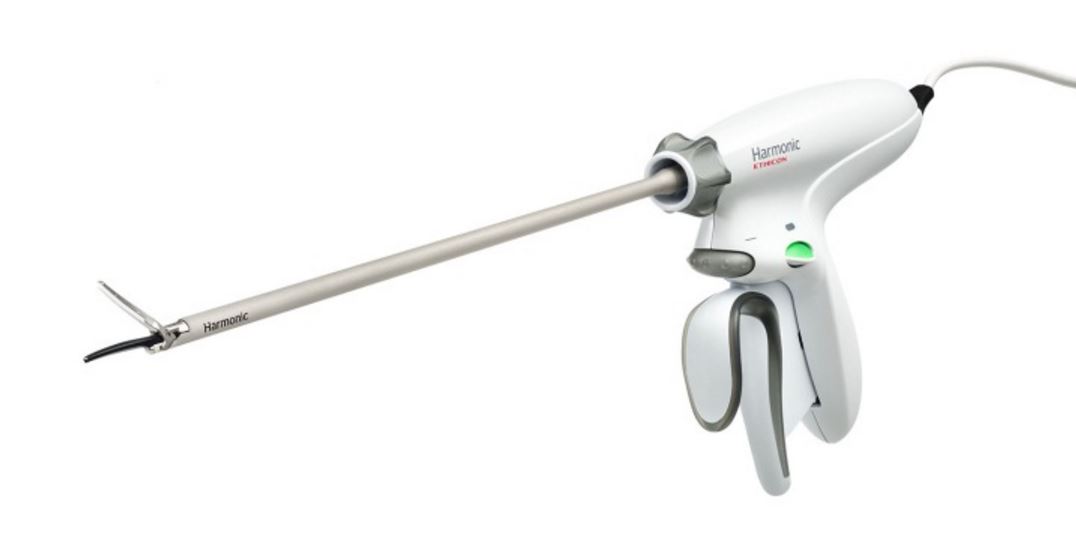
The Harmonic HD 1000i is being positioned as the company’s flagship offering in a line of a energy devices. The shears are intended for high precision surgical procedures, such as intricate thoracic and colorectal issues.
The complexity and design of the device potentially allows surgeons to reduce the number of instruments required for individual procedures. In particular, company literature notes the unique shape of the shears “mimics a mechanical dissector, which may reduce the need to use a separate dedicated dissecting instrument.”
The promotional material also celebrates the increasing efficiency provided by the Harmonic HD 1000i. The device has a single energy button to simplify the process of energy activation and boasts strong tip grasping, which minimizes tissue slippage and other challenges that can slow down a procedure.
(Image credit: Ethicon)
In a press release, Ethicon US president Andrew Ekdahl says the new device is a direct result of listening to the healthcare professionals who face the toughest surgical challenges every day.
“At Ethicon, we challenged ourselves to identify the unmet needs of our customers by speaking with surgeons to understand their concerns in performing complex surgical procedures including oncology,” says Ekdahl. “Today, we are proud to launch a unique device that will help improve the clinical outcomes of patients, while enabling procedural efficiencies that can benefit the health system. Harmonic HD 1000i is the latest example of our commitment to developing meaningful innovations that reach more patients and restore more lives.”
More information can be found at the Harmonic HD 1000i product page.




