
Jim Brown • Business Unit Manager for Medical Markets • Colder Products Co.
Beginning in the 1990s, concerns grew regarding the proliferation of medical devices fitted with luer connectors and the reports of patient injuries and deaths arising from misconnections. Thus began a many-years process to develop new industry standards for the small-bore connectors used in medical device applications.
The much-anticipated ISO 80369 series of standards for small-bore connectors seeks to address patient injuries and deaths due to device misconnections that can occur when incompatible applications use the same, common small-bore connectors. Luers, by far the most frequently used connectors in medical settings, have contributed to the incidence of dangerous medical connections because they can connect with unintended systems. The Introduction to Part 1 of ISO 80369 (hereafter referred to as ISO 80369-1) describes the problem: “In a coronary care unit there are as many as 40 luer connectors on the medical devices used with a single patient. Therefore, it is not surprising that misconnections are made.”
Why are the new ISO 80369 standards significant for medical device OEMs? The need to improve patient safety is the most obvious answer. Another concern is that medical misconnections expose manufacturers and caregivers to potential liability. There are also growing regulatory and potential legal issues to deal with. California law (HB1867) specifically prohibits the use of IV, epidural, and enteral connections that fit into a connection port other than the intended type beginning in 2016. The European Device Directive, the U.S. FDA, and other medical regulatory agencies around the world are addressing medical misconnections. The release of the ISO 80369 series of standards provides connector clarification and guidance in relation to minimizing medical misconnections.

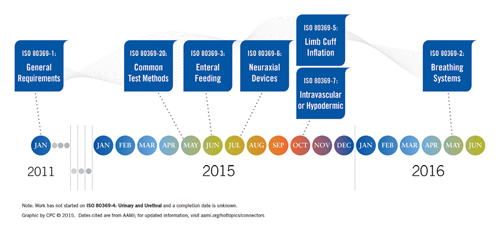
Where ISO 80369 applies to the human system: ISO 80369 Parts 2 to 7 define small-bore connector standards for six application categories. Its goal is to prevent misconnections between unintended devices.
In spirit, ISO 80369 covers medical device small-bore connectors used to make connections between equipment and patients. ISO 80369-1, published in 2011, lays out general requirements for small-bore connectors that facilitate the transfer of liquids and gases in healthcare applications. Parts 2 to 7, in varying stages of development, address six application categories that represent the greatest risks to patients should a medical device misconnection occur. These application categories are: a) breathing system and driving gas, b) enteral and gastric, c) urethral and urinary, d) limb-cuff inflation, e) neuraxial, and f) intravascular or hypodermic applications. An accompanying illustration shows were these parts apply on a patient.
Reducing the domain of luers
Initial work to address medical misconnections focused on defining the applications and the potential risk associated with misconnections. This work quickly showed that too many applications used luers and the widespread use increased the probability of connecting incompatible devices. The first step was limiting the use of the luer to a single application and thus the luer became the connector for vascular access and hypodermic syringes.
Limiting luers to intravenous or hypodermic applications provides clear direction to medical device designers. ISO 80369-7 defines the luer fitting and drives minor changes to the design, primarily defining additional dimensions previously undefined in the old standard, ISO 594-1 and 594-2. Luers made to the ISO 80369-7 standard will connect to luers made to the ISO 594-1 and ISO 594-2 standard, but connectors made to the old standards do not comply with the new ISO 80369-7 standard.
As a result of limiting the use of luers to intravenous or hypodermic applications, other applications such as enteral feeding or neuraxial, which also commonly used luers, needed new connectors designed. The result of this effort is nearing completion with defined connector standards for several of these other applications. Whether or not a current medical device uses luer connectors, or finds its application defined in this new series of standards, product designers should understand the role and recommendations of ISO 80369 in relation to any medical device using a small-bore connector. This article discusses what ISO 80369 defines or will define, and it provides an action plan for assessing the risks of connectors already in use or being considered for use on medical devices and delivery systems.
 Beyond the luer – implementing ISO 80369
Beyond the luer – implementing ISO 80369
Product designers working on devices used in the six defined application categories called out in ISO 80369-2 through 7 will want to become familiar with the general requirements outlined in the first part of the new standard. However, ISO 80369-1 also provides guidance for designers of medical devices for the hundreds of applications outside the categories defined in the new standard. Two sections of ISO 80369-1, in particular, will help designers whose product incorporates fluid handling and small-bore connectors:
- The terms and definitions in Section 3
- The mechanical test for verifying non-interconnectable characteristics in Annex B.
First, Section 3 offers the following definitions that can help product engineers begin designs or select connectors for their medical devices in the context of ISO 80369:
- Small-bore: inner fluid pathway of a connection with a less than 8.5-mm diameter.
- Rigid material: material with a modulus of elasticity either in flexure or in tension greater than 35,000 kg/cm2
- Semi-rigid material: material with a modulus of elasticity either in flexure or in tension between 700kg/cm2 and 35,000 kg/cm2
- Non-interconnectable: having characteristics which incorporate geometries or other characteristics that prevent different connectors from being connected

A variety of small-bore connectors is available for medical device and system applications not specifically defined by ISO 80369. Shown above are product options from CPC that offer the performance and material attributes defined by ISO 80369.
Whether devices in the six defined application categories must use a standard connector can be somewhat confusing. ISO 80369-1 permits use of alternative small-bore connectors as long as they consist of rigid or semi-rigid materials and provide objective evidence that they do not interconnect with standard connectors specified in the six high-risk application categories.
Annex B discusses how to test a connector to provide the required objective evidence that it will not interconnect with the defined, standard connectors. This section includes an assessment for non-interconnectable characteristics of small-bore connectors based on their inherent design and dimensions. There is an important consideration when testing a connector not defined in the standard: The testing will provide results for the defined connectors but does not address other connectors in the market, and other connectors or applications may be added to the series in the future. “Mechanical tests for verifying non-interconnectable characteristics,” from Annex B of ISO 80369-1, summarizes the steps as follows:
a. Condition the connectors for your device to be assembled at 23°C and a relative humidity of 50%.
b. Assemble the small-bore connectors to the test connector by applying an axial force at a rate of approximately 10 N/s – not to exceed 70 N – that does not visibly damage either connector and with a torque not to exceed 0.12 Nm to a limit of no more than 90°.
c. Rotate threaded connectors in a clockwise manner, if applicable.
d. Hold the maximum assembly force for no less than 10 seconds.
e. After 10 seconds of engagement, without activation of any latch or disengagement mechanism,
apply an axial force of separation to the assembled connectors to a maximum of 0.02 N.
f. Verify that the connectors disengage.
g. For threaded connectors, apply steps a. to f. by assembling connectors in a counterclockwise
manner, if applicable.
h. Repeat procedures a. through b. for every potential assembly surface.
Regardless of whether a medical device falls within ISO 80369’s six defined application categories, device manufacturers will want to assess both the physical characteristics of their connectors (according to the terms defined in Section 3) and the non-interconnectable characteristics (according to Annex B).
ISO 80369 goes on to provide one additional type of guidance for assessing small-bore connectors with the performance test methods described in Part 20. This section delineates procedures and formulas for determining performance attributes, such as leakage, resistance to separation from axial load and resistance to overriding. The guidance defines how tests should be conducted but not what values should be achieved. These requirements are found in the documents that define the connector for the specific application (Parts 2 to 7).Even after conducting the assessments outlined above, however, the only connectors that can be determined to fully comply with ISO 80369 series will be those defined in Parts 2 to 7. Connectors for other applications not specifically covered cannot claim compliance with ISO 80369 series because documented standards for those connector applications to demonstrate compliance with do not exist. However, undertaking a risk analysis provides the final and most important analysis for decision-making in relation to any connector change or a new connector selection, regardless of the application.
Risk assessment – the gold standard for mitigating misconnections
The FDA recently published final guidance for one of the defined application categories covered by ISO 80369, that of enteral applications. In its final document titled Safety Considerations to Mitigate the Risk of Misconnections with Small-bore Connectors Intended for Enteral Applications, the FDA advises manufacturers to design and test their products based on standards that specify dimensions and requirements and test methods that support the performance requirements. In plain language, manufacturers must assess the risk of misconnections for their particular device, and then decide if the risk is acceptable.
As with many risk assessments, conducting one in relation to a device connector has three purposes: to determine usability, safety, and regulatory compliance. In many instances, identifying possible hazards associated with the design in normal and fault conditions falls on the shoulders of manufacturers while, typically, the healthcare industry operates under the principle of “safety under single fault conditions,” meaning a single fault should not impose the risk of loss of life. The risk of associated hazards should be, when deemed unacceptable, reduced to acceptable levels by appropriate means. For the purposes of this discussion, the diagram below will guide the examination of risk management as it relates to medical device connectors:
- Start with the selected connector. Risk mitigation begins by answering several questions. Are there any hazards inherent in the connector design or materials? Is the connector easy to operate correctly by professionals as in a hospital setting, and intuitive enough for non-professionals, such as home healthcare providers or family members?
- What other devices are being used in the same environment that may have an interconnectable connector? What is the likelihood of connections being made between these incompatible devices?
- What is the function of the device or machine being connected? Is the fluid transfer under pressure or a vacuum? What type of fluid is being transferred?
- How could a misconnection happen? How frequently is this likely to occur? It is important to consider the environment in which the device will be used. For instance, a blood-pressure cuff in a doctor’s office or supermarket may present a lower risk of misconnection compared to a blood-pressure cuff used in hospitals with IV lines, enteral lines, and others immediately around the patient.
- If a misconnection did occur, what are the possible consequences?
- What level of risk is acceptable for the assessing organization?
Final thoughts
The original CEN Forum Task Group that investigated medical misconnections and the role of luers followed the established medical device industry principle of “safety under single fault conditions” when it recommended limiting the use of luers to IV applications. 3 A later ISO document published in 2006 expanded on the principle with: “In selecting the most appropriate solutions, the manufacturer should apply the following principles in the following order: 1) Identify hazards and the associated risks arising from the intended use and foreseeable misuse; 2) Eliminate or reduce risks as far as possible (inherently safe design and construction).” 4
This emphasis on risk mitigation remains good advice today. Even with ISO 80369’s connector standards for six defined application categories taking shape, the deliberate and documented analysis of risk continues as the OEM device designer and manufacturer’s best guidance in relation to connector decisions for diagnostic, monitoring and drug delivery systems.
For further study:
1: ISO 80369-1:2010(E), Annex A, Rationale
2: FAQs About Small-bore Connectors and Tubing Misconnections, AAMI, Oct. 15, 2013
3: CEN Report CR 13825, 2000
4: ISO/TR 16142:2006, Essential Principle A.1.2


![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)


