From the humble origins of the standard polypropylene mesh, new product development in hernia repair has continued to embrace the potential of resorbable implantable solutions. Over the last decade, composite mesh, non-adhesive PTFE mesh, and resorbable barrier layer mesh have become prerequisite for laparoscopic intraperitoneal repair. Biologic mesh derived from natural tissue stormed the market several years ago, and despite substantive concerns over compromised strength and high cost, it remains a prevalent choice in the treatment of Grade 3 and 4 hernias. Now, however, fully resorbable synthetic meshes are capturing the attention of the market. This article considers the evolution of hernia repair product development and its influence in driving innovation in resorbable implantable solutions.
The global hernia repair market is estimated to be worth almost four billion dollars, with a compound annual growth rate of ca. 8%. Inguinal hernia repair represents almost 75% of this market, with treatment predominantly using synthetic mesh, made from either polypropylene or PET. Despite representing a smaller portion of the market, incisional (ventral) hernia repair is fueling much of the growth in the market overall, driven by a number of demographic factors, such as rising obesity, along with a greater number of elective surgeries, physician training, etc. It is also witnessing a surge in product innovation aimed at improving procedural outcomes in this challenging environment.
Records dating back to the very origins of civilization highlight the evolution of hernia repair treatment, both surgical and non-surgical. What could be classed as modern surgical techniques began with pioneering surgeons, such as Bassini, Shouldice and Halsed in the 19th and 20th centuries. However, it wasn’t until the early 1990’s that the first laparoscopic incisional hernia repair was recorded. To overcome the challenges in modern incisional hernia repair, both open and laparoscopic, considerable development has taken place to improve upon the conventional standard knitted mesh. A significant proportion of incisional hernia repairs, including most laparoscopic repairs, involve placement of mesh in the intraperitoneal space, which can lead to visceral adhesions of the mesh to the bowel. Entering the 21st century, the adoption of advanced processing technologies and biomaterials has led to significant progress in new product development, with an increasing trend in the use of resorbable biomaterials.
The introduction of composite and enhanced standard knit mesh addresses the issue of visceral adhesions and improves product performance. One form of composite mesh integrates resorbable and non-resorbable fibers within the one knit structure. This provides superior mechanical performance upon immediate implantation, but as surrounding tissue begins to heal, the resorbable material disappears. The remaining non-resorbable mesh provides long term support to surrounding tissue but with minimal material.
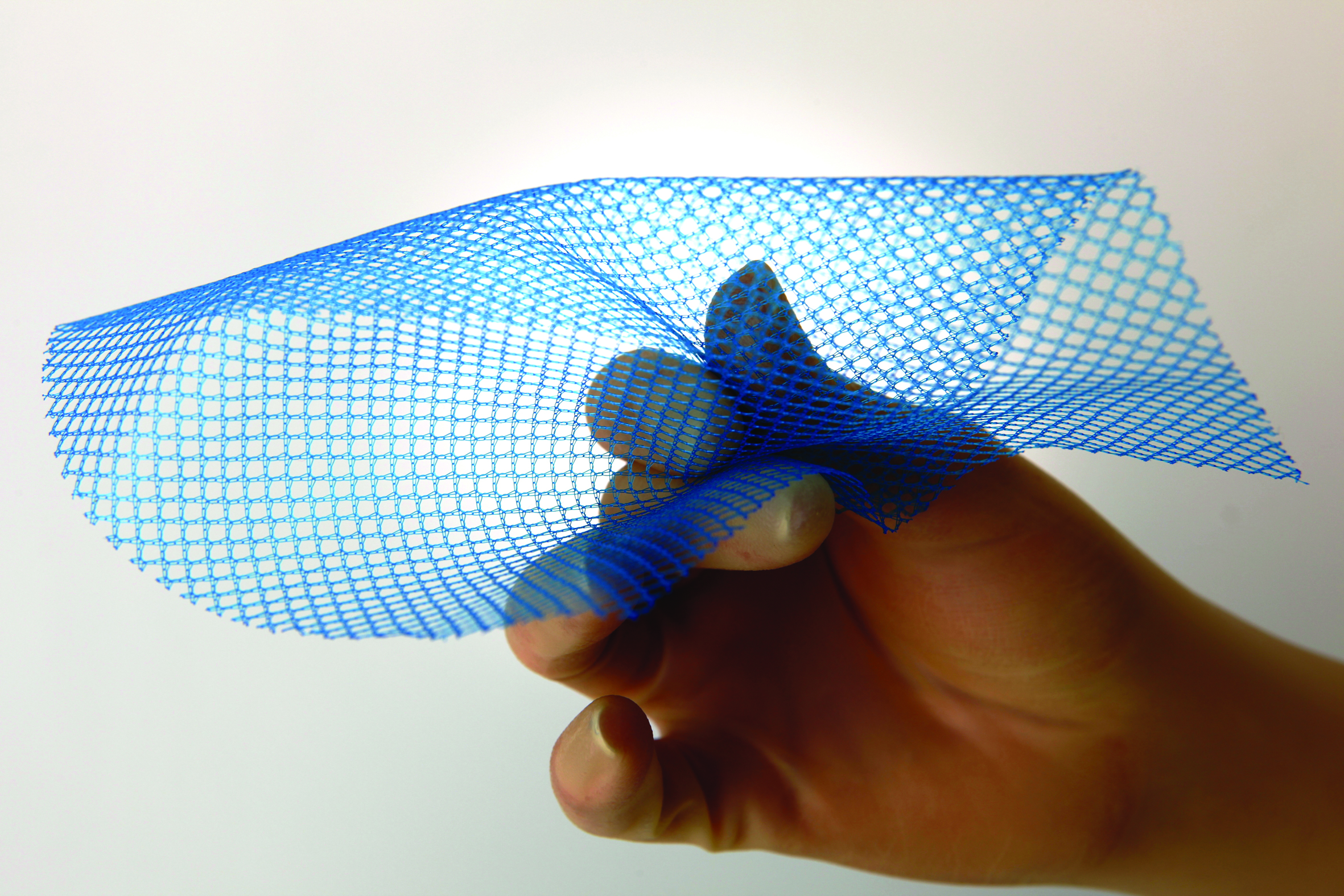
Fig. 1—Vitamesh Blue is a macroporous condensed polypropylene cPP mesh
Most composite meshes, particularly for the treatment of incisional hernia repair, are developed in multi-layered designs. On one side, a macroporous synthetic mesh facilitates tissue ingrowth to provide long term mechanical reinforcement, while the visceral side, facing the bowel, is treated with a non-adhesive barrier to inhibit adhesions. Once scar tissue forms around a mesh, the risk of visceral adhesions is significantly reduced, and resorbable barriers are an ideal candidate to fulfill this temporary function. Medtronic’s Parietex mesh was one of the first composite meshes on the market and it remains one of the most popular. The resorbable film is composed of glycerol, polyethylene glycol, and porcine collagen. Other resorbable materials, such as oxidised cellulose and polyglecaprone, are used in Ethicon’s Proceed and Ultrapro meshes respectively.
The many perceived benefits afforded by laparoscopic ventral hernia repair, in the form of reduced tissue trauma, less pain, faster recovery times, reduced infection and recurrence rates, has led to exponential growth in this approach. To effectively deliver large mesh laparoscopically, Proxy Biomedical developed a proprietary condensing process, which increases the strength of the mesh, while providing a smooth surface and a lower profile to facilitate delivery through a trocar (See Fig. 1 Proxy Biomedical VitaMesh). Similarly, the company provides resorbable barrier layers for mesh from 20-50 micron (0.001 – 0.002”) to ensure low profile composite mesh solutions for large laparoscopic hernia repair (see Fig. 2).
Biologic grafts are now an established resorbable solution for hernia repair. Derived from natural tissue sources, they form acellular collagen matrices that are implanted to encourage native tissue ingrowth. There are numerous biologic meshes on the market, from companies such as Medtronic, Integra, and Acelity. Extensive processing is required to make them fit for use, contributing towards a price that is a multiple of conventional polypropylene mesh and even composite designs. Due to their higher cost, along with mixed clinical evidence supporting superior outcomes, the use of biologics has been primarily reserved for highly complex, Grade 3 and 4 hernias, which are infected or at risk of infection. Another factor limiting the wider adoption of biologic mesh is a lower perceived strength for large hernia repair. As a result, hybrid biologic mesh has emerged on the market, such as Cook’s Zenapro, which features a biologic graft with an integrated polypropylene mesh, to ensure long term tissue support.
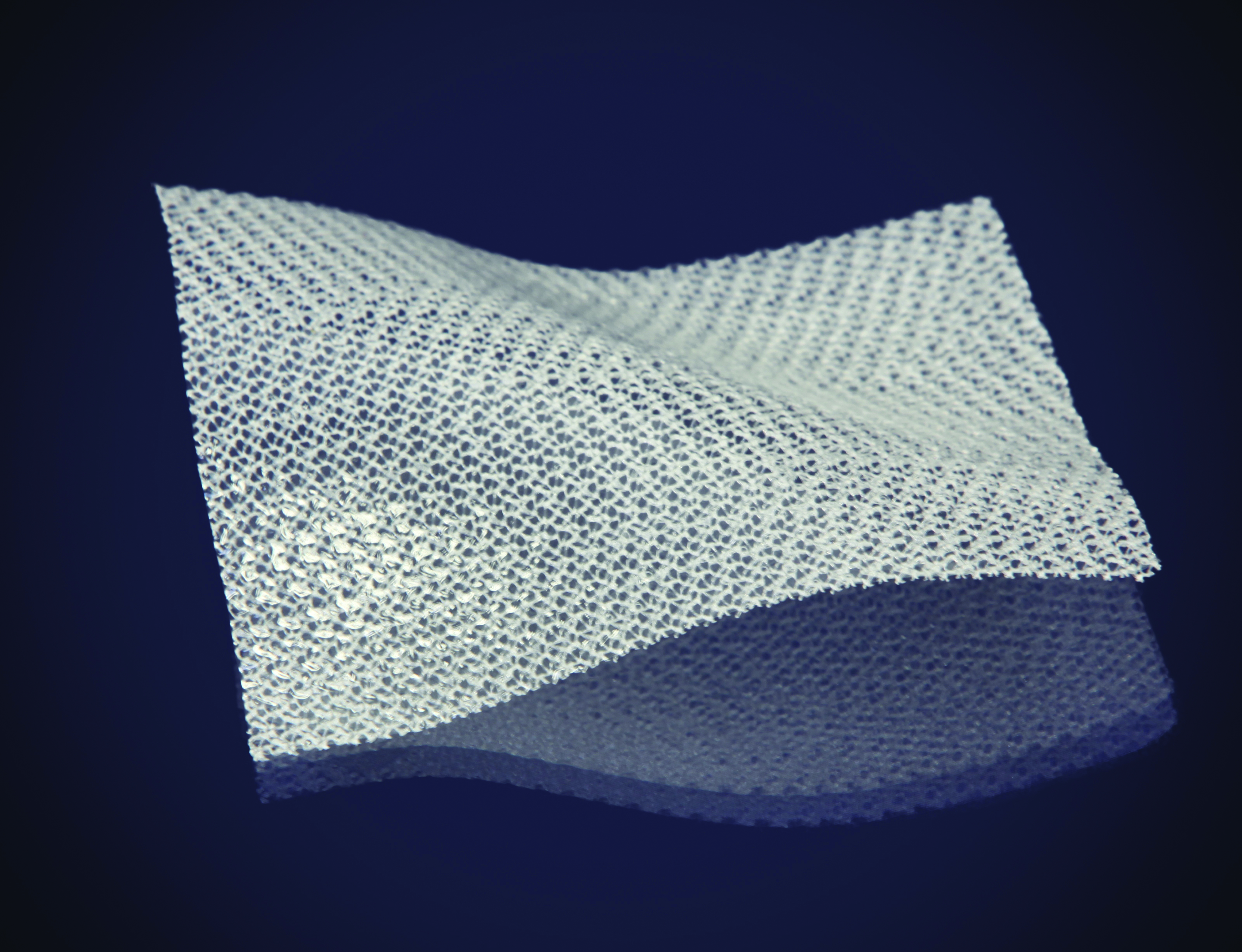
Fig. 2—Mesh with 20 micron resorbable barrier
To overcome some of the challenges with biologic grafts, while maintaining their significant advantages in the treatment of complex hernia, synthetic resorbable mesh has emerged on the market in recent years. There are three companies currently offering solutions, Bard – Phasix mesh, WL Gore – BioA, and Novus Scientific – TIGR surgical mesh. Both Phasix and TIGR resemble conventional mesh, but use resorbable fibers, while BioA features a fibrous non-woven matrix. The most significant challenge for synthetic resorbable mesh, similar to biologic grafts, is to offer sufficient strength and support throughout the time it takes for tissue to integrate and heal around the implant. These meshes have been well received in the market, offering a more cost effective alternative to biologic grafts.
The leading market players in hernia repair now aspire to provide well rounded product portfolios to suit all incisional hernia types and treatment methods. As a result, greater patient populations can be treated with improved clinical outcomes and lower repeat procedures. The use of standard flat sheet mesh for small onlay and inlay treatment has evolved to include composite mesh, biologic grafts, synthetic fully absorbable, and hybrid mesh. This evolution has been possible with the effective use of resorbable materials, which continues to fuel new product innovation in the market.
In our experience, resorbable polymers are playing an increasing role in the evolution of companies’ mesh product portfolios. As we look to the future of hernia repair, the target for product designers is ensure complete durability, minimal pain and elimination of infection. Next generation resorbable hernia repair products are likely to include antimicrobial characteristics, lower profiles to facilitate laparoscopic repair of large hernias, stronger resorbable mesh with improved durability, and adhesive properties to facilitate procedural placement. Also, dimensionally speaking, there are already numerous meshes tailor made for specific treatment methods or hernia indications, such as parastomal and umbilical mesh, and we’re likely to see this trend continue with fully resorbable solutions. New product development in this space will be achieved through continued biomaterial innovation, novel processing techniques and sophisticated materials management systems.
As witnessed in the past, the achievements made to date in hernia repair are likely to be translated into implantable solutions for wider range of soft issue and sports medicine applications, as well as sophisticated vascular devices. Despite being one of the oldest forms of surgical treatment, new product development in hernia repair is stimulating innovation across a wide spectrum of treatment areas, ensuring a brighter future in patient care.
This article appeared in the March 2016 print edition of MDT.




