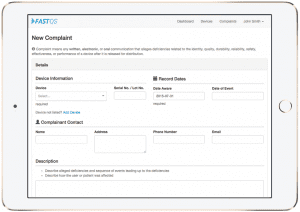
The complaint management solution features include compliancy, trending codes, complaint network, RMA link, and quick setup.
FastQS recently announced the launch of its modern cloud-based Complaint Management Solution designed specifically for the medical device industry. With FastQS, medical device companies can easily process complaints paperlessly and compliantly.
Robust complaint management is a requirement under the Code of Federal Regulations Title 21 and is enforced by the FDA through regular inspections. In the past 5 years, complaint handling processes have consistently been a top issue during FDA Inspections, attributing to numerous 483s and Warning Letters filed against noncompliant device manufacturers.
An overwhelming majority of medical device companies utilize a highly-manual, paper-based complaint handling process. Coupled with the risk for data loss and security concerns, this becomes a deceptively expensive option. The increasing adoption of cloud enables FastQS to offer an easy-to-use and affordable solution. Since the application was designed with the regulations in mind, very little customization or maintenance is required.
FastQS has taken industry best practices from regulatory experts and put them into a first-rate enterprise solution. “FastQS is about changing the way we see quality in the medical device industry. We believe that providing affordable regulatory solutions will drive improved patient safety and reduce overall healthcare costs.” said Alvin Tai, CEO and co-founder of FastQS.
FastQS Features Include:
- Compliancy: FastQS is compliant to FDA CFR Title 21 Part 820 – Quality System Regulations, Part 803 – Medical Device Reporting and Part 11 – Electronic Records; Electronic Signatures.
- Trending Codes: Assign relevant codes to each complaint for advanced trending capabilities. Identify quality issues quickly and accurately.
- Complaint Network: Link complaints to a similar “master” complaint to avoid repeating unnecessary investigations.
- RMA Link: Connect with an externally generated RMA number to link complaints with returned material.
- Quick Setup: FastQS provides all the necessary procedure templates, software validation documentation and guidance to quickly integrate with any quality system.
FastQS
www.fastqs.com




