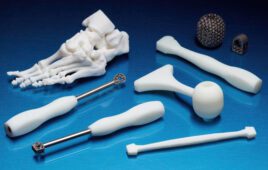
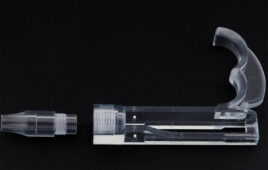
[Image from the Cleveland Clinic]
Cleveland Clinic physician Tom Gildea developed the stents to keep open the airways of patients with serious breathing disorders caused by inflammation, trauma, tumors and other masses.
Airway stents come in a limited number of sizes and shapes and are typically designed for larger airways, according to the Cleveland Clinic. Patient anatomies differ, making it difficult to get an exact fit. If a stent doesn’t fit well, it can kink or bend and cause airway complications.
The patient-specific stents developed at the Cleveland Clinic are designed using CT scans and 3D visualization software. The molds for the stents are 3D-printed and injected with medical-grade silicone that allows them to fit perfectly in different anatomies.
“Breathing is something many people take for granted, but for many of these patients, every breath can be a struggle. It’s been gratifying to see patients receiving the customized stents feeling relief right away,” Gildea, who is the head of bronchoscopy at the Cleveland Clinic, said in a news release. “We are excited to be able to bring this technology to more patients across the country and grateful for the patients and donors who have worked with us to help pioneer this technology.”
Cleveland Clinic suggests that patient-specific silicone stents have the potential to be more tolerable than traditional silicone stents, which have to be frequently changed or cleaned because of a poor fit. The 3D-printed, patient-specific stents lasted a year versus 90 days with stock stents, according to studies. They also have shorter procedure times and improved patient-reported symptoms.
Patient-specific products achieved with 3D printing made the list of 2019 disruptors outlined by the Cleveland Clinic at the 2018 Innovation Summit.
“3D printing is a remarkable tool,” Gildea, who presented the innovation at the summit, said in 2018.
With the new patient-specific stents, the Cleveland Clinic plans to open a subsidiary known as VisionAir solutions to bring more personalized medical devices to interventional pulmonologists. The Cleveland Clinic expects to begin providing personalized stents to patients in a controlled launch by the end of the first quarter of 2020.