The U.S. Food and Drug Administration on Tuesday approved the first fully absorbable stent to treat coronary artery disease.
The Absorb GT1 Bioresorbable Vascular Scaffold System (BVS), manufactured by Abbott Vascular, Santa Clara, CA, releases the drug everolimus to limit the growth of scar tissue and is gradually absorbed by the body in approximately three years.
“The FDA’s approval of the Absorb GT1 BVS offers a new treatment option for individuals who are candidates for angioplasty, but would prefer an absorbable device rather than a permanent metallic coronary stent,” said Bram Zuckerman, M.D., director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health.
But not so fast, say some experts.
The newly approved Absorb stent comes with one important caveat: It hasn’t yet been shown to be safer than older metal implants.
Coronary artery disease, an artery-narrowing condition, causes about 370,000 U.S. deaths each year, according to government figures. The new stent is designed to gradually dissolve.
The device is scheduled to be implanted at HonorHealth Scottsdale Shea Medical Center in Scottsdale, AZ on July 6 in what may represent the first U.S. patient to receive the stent.
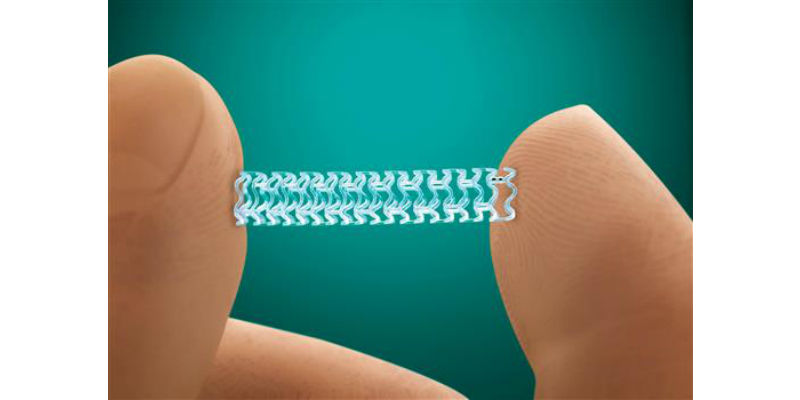
This photo provided by Abbott Laboratories shows the company’s Absorb stent. On Tuesday, July 5, 2016, the Food and Drug Administration approved the slowly-dissolving medical implant for treating clogged arteries. The new stent is designed to dissolve over three years. Currently-available stents are permanent, mesh-wire tubes that hold open arteries after a procedure used to clear fatty plaque. (Credit: Abbott Laboratories via AP)
Currently available stents are permanent, mesh-wire tubes that hold open arteries after a procedure used to clear fatty plaque.
Experts describe Abbott’s device as an important step in finding a better approach to treating the leading cause of death in the U.S.
“This is presumably a better technology going forward, at least that’s the theory, but it will take years to prove,” said Dr. George Vetrovec, professor emeritus at Virginia Commonwealth University. Vetrovec was part of an FDA advisory panel of cardiologists who overwhelmingly endorsed the device in March.
The Absorb stent, already sold in Europe and Asia, is made of a degradable material that’s designed to stay intact for one year then break down over the next two years.
Doctors at HonorHealth moved quickly to schedule the first U.S. patient, who was not identified. But, members of the media have been invited to attend the initial procedure and the hospital group is planning to arrange interviews with the doctors and patient as early as Thursday.
“This is a game changer in coronary disease management,” said David G. Rizik, MD, an HonorHealth interventional cardiologist who was a principal investigator for the device during clinical trials at HonorHealth Research Institute. Rizik will perform the first FDA-approved implant in the United States. “It’s the next big advance.”
While other medical professionals caution that only time will tell for sure, HonorHealth, in a statement issued Tuesday, said the new stent performs as well as or better than its predecessor, the drug-coated metal stent, but may have greater long-term advantages for the patients.
“With the fully dissolving stent, we believe there will be less scarring or inflammation,” Rizik said. “The artery can return to a more natural state, expanding and contracting with exercise or exertion. A metal stent is permanent and restricts motion by caging the vessel, giving it no opportunity to grow or enlarge.”
Rizik noted that the fully dissolving stent is a desirable option for patients who prefer not to have a permanent metal implant in their body for the rest of their lives.
Use of metallic stents surged in the early 2000s as a treatment for people who suffered a heart attack or experienced chest pain caused by clogged arteries that restrict blood flow. They are still implanted in about 850,000 U.S. patients annually.
But doctors have scaled back their use due to safety concerns, insurance cost-cutting and evidence that they are overused. Studies in 2007 and 2008 suggested that stented arteries faced a higher risk of blood clots, potentially triggering heart attack a year or more down the road. Results of another five-year study showed that patients who received stents to treat chest pain fared about as well as those treated with drugs.
Amid these concerns, Abbott and others began developing dissolving stents that would slowly melt away like stitches, presumably reducing complications.
In the company study submitted to the FDA, patients who got Absorb fared about as well as those receiving Abbott’s older metal stent after one year. But heart-related complications were actually slightly higher with Absorb — 7.8 percent of patients, versus 6.1 percent of patients with the metal stent. That 1.7 percent difference is not considered statistically significant.
Other complications with the new device include allergic reactions, infections and internal bleeding.
The fully dissolving stent is made of a material commonly used in such medical implants as dissolvable sutures and dissolvable bone screws. The stent is coated with a medication that reduces inflammation and tissue growth to help prevent further blockage of the artery.
HonorHealth cardiac researchers spent the last 10 years working with the manufacturer to bring the technology to market, performing clinical trials and publishing numerous scientific papers that eventually helped lead to FDA approval. Coronary artery disease is the most common type of heart disease.
Nationally, doctors who studied Absorb said it may take several years before its advantages become clear. Long-term safety results aren’t expected until 2020.
“We have good theoretical reasons to believe that by getting rid of the stent, and allowing the coronary artery to restore its normal shape, that will prevent many of those late events,” said Dr. Gregg Stone, of Columbia University Medical Center, who helped conduct the pivotal trial of Absorb. Stone was not paid by Abbott for his work on the trial.
Some Wall Street analysts are betting that products like Absorb will allow companies to raise prices and boost revenue.
“The key for the stent market will be whether pricing returns to rational levels and the success of the new bio-absorbable stent platforms,” states Evercore ISI analyst Vijay Kumar, in an investment note. He says a “perfect storm of events,” including safety concerns and the economic downturn led Abbott and competitors Medtronic Inc. and Boston Scientific Corp. to slash prices in recent years.
Stent sales fell roughly 30 percent to $4.1 billion in 2014 from 2006 levels, according to Evercore.
Abbott said it does not disclose the price of its stents. Hospitals often bill $30,000 for stenting procedures, which includes the costs of the stent, medical staff and other equipment and services.
Chicago-based Abbott controls a little more than a third of the U.S. stent market. More than a dozen medical device makers are working on their own dissolving stent technologies.
___
(Story credits: Matthew Perrone, AP Health Writer; Linda A. Johnson, AP Medical Writer; HonorHealth Scottsdale Shea Medical Center; Abbott Laboratories; U.S. Food and Drug Administration)




