The U.S. Food and Drug Administration today approved the first fully absorbable stent to treat coronary artery disease. The Absorb GT1 Bioresorbable Vascular Scaffold System (BVS), which releases the drug everolimus to limit the growth of scar tissue, is gradually absorbed by the body in approximately three years.
“The FDA’s approval of the Absorb GT1 BVS offers a new treatment option for individuals who are candidates for angioplasty, but would prefer an absorbable device rather than a permanent metallic coronary stent,” says Bram Zuckerman, M.D., director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health.
Coronary heart disease is responsible for about 370,000 deaths each year in the U.S., according to the National Heart, Lung, and Blood Institute. The condition develops when cholesterol-containing deposits build up and narrow the coronary arteries, decreasing blood flow to the heart. This can cause chest pain (angina), shortness of breath, fatigue or other heart disease symptoms. Doctors often treat coronary artery disease with a procedure called angioplasty to widen the artery using a metal stent. Scar tissue can form within the stent causing the artery to narrow again (restenosis). Drug-eluting stents temporarily release a drug, typically for a few months after stent placement, to combat the formation of scar tissue.
The Absorb GT1 BVS is manufactured from a biodegradable polymer called poly(L-lactide), which is similar to materials used in other types of absorbable medical devices, such as sutures. The device’s absorption by the body gradually eliminates the presence of foreign material in the artery once the stent is no longer needed. After absorption, there are only four very small platinum markers embedded in the walls of the artery, which help cardiologists identify where the Absorb GT1 BVS was originally placed.
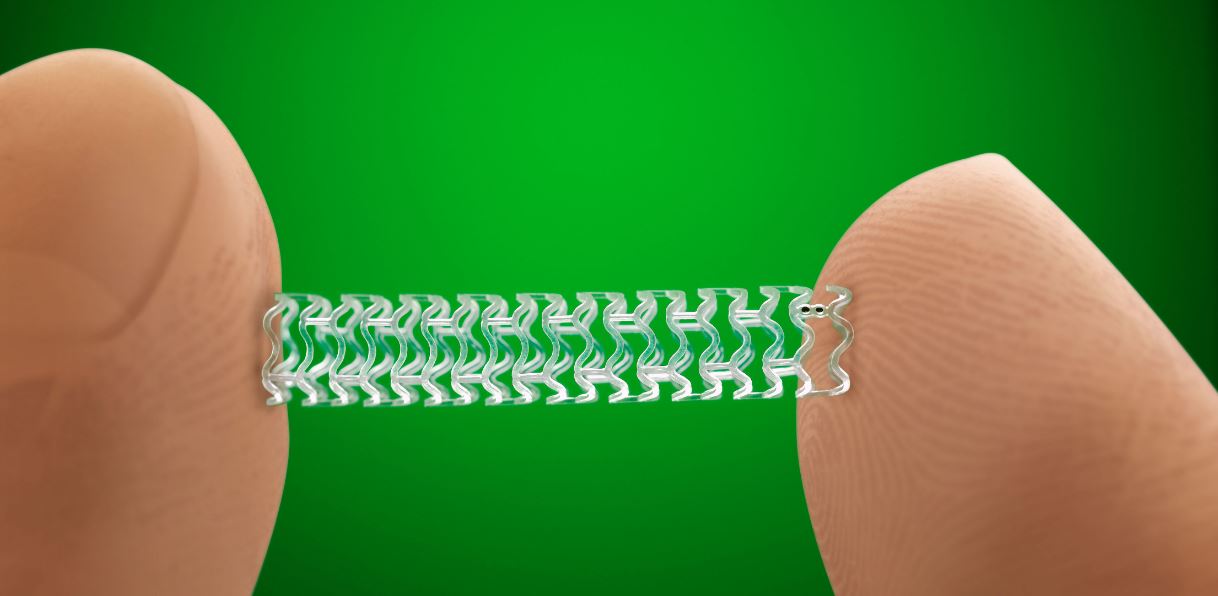
(Image credit: Abbott Vascular promotional image)
In approving the Absorb GT1 BVS, the FDA evaluated data from a randomized trial of 2,008 patients, which compared the rate of major adverse cardiac events between the Absorb GT1 BVS and a drug-eluting metallic stent. After one year, the Absorb GT1 BVS group showed a major cardiac adverse event rate of 7.8 percent, which was clinically comparable to the rate of 6.1 percent observed in the control group. In addition, after one year, the rate of blood clots forming within the devices was 1.54 percent for the Absorb GT1 BVS and 0.74 percent rate for the control.
Possible adverse events that may be associated with the procedure to insert the Absorb GT1 BVS or with the Absorb GT1 BVS itself include allergic reactions to materials in the device or medications used during the procedure, allergic reaction to the drug everolimus, infection or irritation at the catheter insertion site, internal bleeding, the development of abnormal connections between arteries and veins, embolism, or other coronary artery complications that may require medical intervention and that could lead to death.
The Absorb GT1 BVS is contraindicated for patients who have a known hypersensitivity or allergy to everolimus or the materials used in the device, such as poly(L-lactide), poly(D,L-lactide), or platinum. It is also contraindicated for those who are not candidates for angioplasty, have sensitivity to contrast, or who cannot take long-term aspirin therapy along with other blood-thinning medications (antiplatelet agents).
The Absorb GT1 BVS is manufactured by Abbott Vascular in Santa Clara, California.




