The U.S. Food and Drug Administration has approved a new implantable device for patients with moderate to severe central sleep apnea. (Credit: Michael Symonds/Wikimedia)
The U.S. Food and Drug Administration announced it has approved a new implantable device for patients with moderate to severe central sleep apnea.
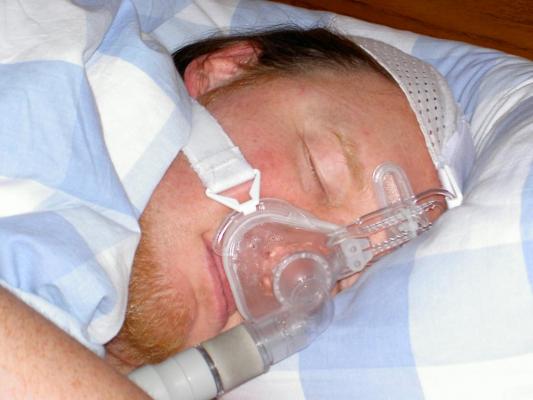
The implantable device offers sleep apnea patients an alternative to continuous positive airway pressure, or CPAP, a mask connected to a motor that provides air through a tube to keep breathing airways open.
Sleep apnea causes individuals to have one or more pauses in breathing, or shallow breathing, during sleep. Central sleep apnea happens when the brain fails to send signals to the diaphragm to breathe, causing a person to stop breathing during sleep for 10 seconds or more before restarting again.
“This implantable device offers patients another treatment option for central sleep apnea,” Dr. Tina Kiang, acting director of the Division of Anesthesiology, General Hospital, Respiratory, Infection Control, and Dental Devices in the FDA’s Center for Devices and Radiological Health, said in a press release. “Patients should speak with their health care providers about the benefits and risks of this new treatment compared to other available treatments.”
The FDA approved the Remedē System, an implantable device that stimulates a nerve located in the chest to send signals to the diaphragm to stimulate breathing, offering another option for sleep apnea patients.
The system contains a battery pack surgically placed under the skin in the upper chest area and small, thin wires that are inserted into blood vessels in the chest near the phrenic nerve to stimulate breathing.
The FDA analyzed data from 141 patients with the reducing apnea hypopnea index, or AHI, and found that, with the system, the Remedē System reduced AHI by 50 percent over a six-month period in 51 percent of patients. AHI was reduced by just 11 percent in patients without the implant.




