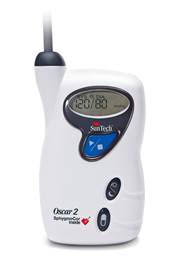
 SunTech Medical and AtCor Medical have received clearance from the Food and Drug Administration for U.S. distribution of their jointly developed Oscar 2 ambulatory blood pressure monitor (ABPM) with SphygmoCor Inside. The Oscar 2 with SphygmoCor Inside provides physicians, researchers and pharmaceutical companies with non-invasive measures of central arterial pressure waveforms, central aortic blood pressures and arterial stiffness indices, as well as the highly-accurate data associated with a 24-hour ABPM study.
SunTech Medical and AtCor Medical have received clearance from the Food and Drug Administration for U.S. distribution of their jointly developed Oscar 2 ambulatory blood pressure monitor (ABPM) with SphygmoCor Inside. The Oscar 2 with SphygmoCor Inside provides physicians, researchers and pharmaceutical companies with non-invasive measures of central arterial pressure waveforms, central aortic blood pressures and arterial stiffness indices, as well as the highly-accurate data associated with a 24-hour ABPM study.
In addition to AtCor Medical’s proprietary SphygmoCor Inside technology, the Oscar 2 uses SunTech Medical’s motion-tolerant technology to eliminate ambulation and noise artifacts that cause failed readings and the need for repeated studies. A comfortable, form-fitting sleeve design ensures proper cuff position throughout the study as well as consistent, accurate readings. Patented sub-diastolic waveform measurement delivers a fully-featured physiological waveform with accurate augmentation index in all patients 18 and over. Additionally, the monitor is designed to be used with SunTech’s AccuWin Pro 4 software, allowing data to be directly exported to a computer database for efficient programming, measurement analysis, and physician interpretation and report.
The product was developed as part of a multi-year alliance between SunTech Medical and AtCor Medical. It received CE Mark certification in May 2015 and is being marketed by AtCor Medical and SunTech Medical through joint and exclusive sales channels around the world including the Americas, Australia, New Zealand, the European Union and selected Asian countries.
The Oscar 2 with SphygmoCor inside has been independently tested to meet the accuracy and performance requirements of the BHS, ESH and AAMI: SP10.
SunTech Medical
www.suntechmed.com
AtCor Medical
www.AtCormedical.com




